Efficient Biosynthesis of Salidroside via Artificial in Vivo enhanced UDP-Glucose System Using Cheap Sucrose as Substrate
- PMID: 38799314
- PMCID: PMC11112596
- DOI: 10.1021/acsomega.4c02060
Efficient Biosynthesis of Salidroside via Artificial in Vivo enhanced UDP-Glucose System Using Cheap Sucrose as Substrate
Abstract
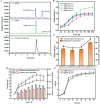
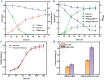
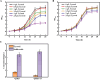
Salidroside, a valuable phenylethanoid glycoside, is obtained from plants belonging to the Rhodiola genus, known for its diverse biological properties. At present, salidroside is still far from large-scale industrial production due to its lower titer and higher process cost. In this study, we have for the first time increased salidroside production by enhancing UDP-glucose supply in situ. We constructed an in vivo UDP-glucose regeneration system that works in conjunction with UDP-glucose transferase from Rhodiola innovatively to improve UDP-glucose availability. And a coculture was formed in order to enable de novo salidroside synthesis. Confronted with the influence of tyrosol on strain growth, an adaptive laboratory evolution strategy was implemented to enhance the strain's tolerance. Similarly, salidroside production was optimized through refinement of the fermentation medium, the inoculation ratio of the two microbes, and the inoculation size. The final salidroside titer reached 3.8 g/L. This was the highest titer achieved at the shake flask level in the existing reports. And this marked the first successful synthesis of salidroside in an in situ enhanced UDP-glucose system using sucrose. The cost was reduced by 93% due to the use of inexpensive substrates. This accomplishment laid a robust foundation for further investigations into the synthesis of other notable glycosides and natural compounds.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Engineering Escherichia coli for Efficient De Novo Synthesis of Salidroside.J Agric Food Chem. 2024 Dec 25;72(51):28369-28377. doi: 10.1021/acs.jafc.4c10247. Epub 2024 Dec 12. J Agric Food Chem. 2024. PMID: 39666864
-
Highly efficient biosynthesis of salidroside by a UDP-glucosyltransferase-catalyzed cascade reaction.Biotechnol Lett. 2024 Apr;46(2):173-181. doi: 10.1007/s10529-023-03453-0. Epub 2024 Jan 6. Biotechnol Lett. 2024. PMID: 38184486
-
Convergent engineering of syntrophic Escherichia coli coculture for efficient production of glycosides.Metab Eng. 2018 May;47:243-253. doi: 10.1016/j.ymben.2018.03.016. Epub 2018 Mar 27. Metab Eng. 2018. PMID: 29596994
-
[Salidroside biosynthesis pathway: the initial reaction and glycosylation of tyrosol].Sheng Wu Gong Cheng Xue Bao. 2012 Mar;28(3):282-94. Sheng Wu Gong Cheng Xue Bao. 2012. PMID: 22712387 Review. Chinese.
-
Salidroside: A review of its recent advances in synthetic pathways and pharmacological properties.Chem Biol Interact. 2021 Apr 25;339:109268. doi: 10.1016/j.cbi.2020.109268. Epub 2021 Feb 20. Chem Biol Interact. 2021. PMID: 33617801 Review.
Cited by
-
Efficient production of hydroxysalidroside in Escherichia coli via enhanced glycosylation and semi-rational design of UGT85A1.Synth Syst Biotechnol. 2025 Mar 6;10(2):638-649. doi: 10.1016/j.synbio.2025.03.002. eCollection 2025 Jun. Synth Syst Biotechnol. 2025. PMID: 40166613 Free PMC article.
References
-
- Marchev A. S.; Dinkova-Kostova A. T.; Gyorgy Z.; Mirmazloum I.; Aneva I. Y.; Georgiev M. I. hodiola rosea L.: From golden root to green cell factories. Phytochemistry Reviews. 2016, 15, 515–536. 10.1007/s11101-016-9453-5. - DOI
LinkOut - more resources
Full Text Sources
