Depletion of donor dendritic cells ameliorates immunogenicity of both skin and hind limb transplants
- PMID: 38799435
- PMCID: PMC11116604
- DOI: 10.3389/fimmu.2024.1395945
Depletion of donor dendritic cells ameliorates immunogenicity of both skin and hind limb transplants
Abstract
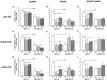
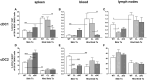
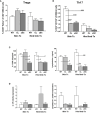
Acute cellular rejection remains a significant obstacle affecting successful outcomes of organ transplantation including vascularized composite tissue allografts (VCA). Donor antigen presenting cells (APCs), particularly dendritic cells (DCs), orchestrate early alloimmune responses by activating recipient effector T cells. Employing a targeted approach, we investigated the impact of donor-derived conventional DCs (cDCs) and APCs on the immunogenicity of skin and skin-containing VCA grafts, using mouse models of skin and hind limb transplantation. By post-transplantation day 6, skin grafts demonstrated severe rejections, characterized by predominance of recipient CD4 T cells. In contrast, hind limb grafts showed moderate rejection, primarily infiltrated by CD8 T cells. Notably, the skin component exhibited heightened immunogenicity when compared to the entire VCA, evidenced by increased frequencies of pan (CD11b-CD11c+), mature (CD11b-CD11c+MHCII+) and active (CD11b-CD11c+CD40+) DCs and cDC2 subset (CD11b+CD11c+ MHCII+) in the lymphoid tissues and the blood of skin transplant recipients. While donor depletion of cDC and APC reduced frequencies, maturation and activation of DCs in all analyzed tissues of skin transplant recipients, reduction in DC activities was only observed in the spleen of hind limb recipients. Donor cDC and APC depletion did not impact all lymphocyte compartments but significantly affected CD8 T cells and activated CD4 T in lymph nodes of skin recipients. Moreover, both donor APC and cDC depletion attenuated the Th17 immune response, evident by significantly reduced Th17 (CD4+IL-17+) cells in the spleen of skin recipients and reduced levels of IL-17E and lymphotoxin-α in the serum samples of both skin and hind limb recipients. In conclusion, our findings underscore the highly immunogenic nature of skin component in VCA. The depletion of donor APCs and cDCs mitigates the immunogenicity of skin grafts while exerting minimal impact on VCA.
Keywords: Th17 immune response; acute cellular rejection; allograft immunogenicity; antigen presenting cells (APCs); conventional dendritic cells (cDCs); mouse models of skin and hind limb transplantation; vascularized composite-tissue allografts (VCA).
Copyright © 2024 Ashraf, Mengwasser, Reutzel-Selke, Polenz, Führer, Lippert, Tang, Michaelis, Catar, Pratschke, Witzel, Sauer, Tullius and Kern.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

