Case report: A highly active refractory myasthenia gravis with treatment of telitacicept combined with efgartigimod
- PMID: 38799457
- PMCID: PMC11116603
- DOI: 10.3389/fimmu.2024.1400459
Case report: A highly active refractory myasthenia gravis with treatment of telitacicept combined with efgartigimod
Abstract
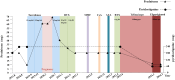
There is always a lack of effective treatment for highly active refractory generalized myasthenia gravis (GMG). Recently, telitacicept combined with efgartigimod significantly reduces circulating B cells, plasma cells, and immunoglobulin G, which brings promising therapeutic strategies. We report a case of a 37-year-old female patient with refractory GMG, whose condition got significant improvement and control with this latest treatment after multiple unsuccessful therapies of immunosuppressants. The new combination deserves further attention in the therapeutic application of myasthenia gravis.
Keywords: case report; efgartigimod; highly active; myasthenia gravis; refractory; telitacicept.
Copyright © 2024 Zhang, Lin, Kuang, Li, Jiang and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
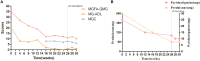

References
-
- Howard JJ, Bril V, Vu T, Karam C, Peric S, Margania T, et al. . Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (adapt): a multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. (2021) 20:526–36. doi: 10.1212/WNL.96.15_supplement.4520 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

