Phosphomethylpyrimidine Synthase (ThiC): Trapping of Five Intermediates Provides Mechanistic Insights on a Complex Radical Cascade Reaction in Thiamin Biosynthesis
- PMID: 38799670
- PMCID: PMC11117688
- DOI: 10.1021/acscentsci.4c00125
Phosphomethylpyrimidine Synthase (ThiC): Trapping of Five Intermediates Provides Mechanistic Insights on a Complex Radical Cascade Reaction in Thiamin Biosynthesis
Abstract
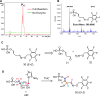
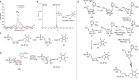
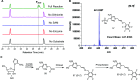
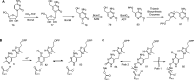
Phosphomethylpyrimidine synthase (ThiC) catalyzes the conversion of AIR to the thiamin pyrimidine HMP-P. This reaction is the most complex enzyme-catalyzed radical cascade identified to date, and the detailed mechanism has remained elusive. In this paper, we describe the trapping of five new intermediates that provide snapshots of the ThiC reaction coordinate and enable the formulation of a revised mechanism for the ThiC-catalyzed reaction.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








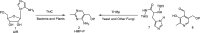
References
-
- Begley T. P.; Ealick S. E.. 7.15 - Thiamin Biosynthesis. In Comprehensive Natural Products II; Liu H.-W., Mander L., Eds.; Elsevier: Oxford, 2010; pp 547–559.
LinkOut - more resources
Full Text Sources
