The role of syntaxins in retinal function and health
- PMID: 38799985
- PMCID: PMC11119284
- DOI: 10.3389/fncel.2024.1380064
The role of syntaxins in retinal function and health
Abstract
The soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein (SNAP) receptor (SNARE) superfamily plays a pivotal role in cellular trafficking by facilitating membrane fusion events. These SNARE proteins, including syntaxins, assemble into complexes that actively facilitate specific membrane fusion events. Syntaxins, as integral components of the SNARE complex, play a crucial role in initiating and regulating these fusion activities. While specific syntaxins have been extensively studied in various cellular processes, including neurotransmitter release, autophagy and endoplasmic reticulum (ER)-to-Golgi protein transport, their roles in the retina remain less explored. This review aims to enhance our understanding of syntaxins' functions in the retina by shedding light on how syntaxins mediate membrane fusion events unique to the retina. Additionally, we seek to establish a connection between syntaxin mutations and retinal diseases. By exploring the intricate interplay of syntaxins in retinal function and health, we aim to contribute to the broader comprehension of cellular trafficking in the context of retinal physiology and pathology.
Keywords: SNARE; retina; retinal disease; synapse; syntaxin.
Copyright © 2024 Tebbe, Kakakhel, Al-Ubaidi and Naash.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
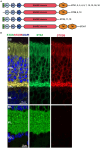
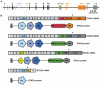
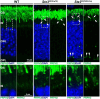

References
-
- Akhrif M., Sabib M., Rouas L., Meskini T., Mouane N. (2021). A rare cause of neonatal diarrhoea: Microvillositary inclusion disease: about a case report. Health 4, 053–056. doi: 10.29328/journal.japch.1001033 - DOI
-
- Al-Yaqoubi S. (2023). Children with microvillus inclusion disease in Oman. Open Acc. Libr. J. 10, 1–9. doi: 10.4236/oalib.1109748 - DOI
Publication types
LinkOut - more resources
Full Text Sources

