Quality by design approach for development and characterization of gabapentin-loaded solid lipid nanoparticles for intranasal delivery: In vitro, ex vivo, and histopathological evaluation
- PMID: 38800014
- PMCID: PMC11127077
- DOI: 10.22038/IJBMS.2024.76281.16511
Quality by design approach for development and characterization of gabapentin-loaded solid lipid nanoparticles for intranasal delivery: In vitro, ex vivo, and histopathological evaluation
Abstract
Objectives: "Quality by Design" (QbD) is a novel approach to product development that involves understanding the product and process, as well as the relationship between critical quality attributes (CQA) and critical process parameters (CPP). This study aimed to optimize the gabapentin-loaded solid lipid nanoparticle formulation (GP-SLN) using a QbD approach and evaluate in vitro and ex vivo performance.
Materials and methods: The GP-SLN formulation was created using the microemulsion method by combining Gelucire 48/16, Tween 80, and Plurol Oleique CC 497. The Box-Behnken experimental design was adopted to investigate the effects of independent factors on dependent factors. The GP-SLN formulation was assessed based on particle size and distribution, zeta potential, morphology, entrapment efficiency, release kinetics, permeation parameters, stability, and nasal toxicity.
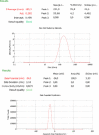
Results: The nanoparticles had a cubical shape with a particle size of 185.3±45.6 nm, a zeta potential of -24±3.53 mV, and an entrapment efficiency of 82.57±4.02%. The particle size and zeta potential of the GP-SLNs remained consistent for 3 months and followed Weibull kinetics with a significantly higher ex vivo permeability (1.7 fold) than a gabapentin solution (GP-SOL). Histopathology studies showed that intranasal administration of the GP-SLN formulation had no harmful effects.
Conclusion: The current study reports the successful development of a GP-SLN formulation using QbD. A sustained release of GP was achieved and its nasal permeability was increased. Solid lipid nanoparticles with optimum particle size and high entrapment efficiency may offer a promising approach for the intranasal delivery of drugs.
Keywords: Box-Behnken design; Gabapentin; Histopathology; Nasal delivery; Permeation; Release kinetics; Solid lipid nanoparticle.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures








References
-
- Thurman DJ, Beghi E, Begley CE, Berg AT, Buchhalter JR, Ding D, et al. Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia. 2011;52:2–26. - PubMed
-
- Cameron A, Bansal A, Dua T, Hill SR, Moshe SL, Mantel-Teeuwisse AK, et al. Mapping the availability, price, and affordability of antiepileptic drugs in 46 countries. Epilepsia. 2012;53:962–969. - PubMed
-
- Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult epilepsy. Lancet. 2006;367:1087–1100. - PubMed
-
- Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: Definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46:470–472. - PubMed
LinkOut - more resources
Full Text Sources
