Prolonged use of nomegestrol acetate and risk of intracranial meningioma: a population-based cohort study
- PMID: 38800110
- PMCID: PMC11127190
- DOI: 10.1016/j.lanepe.2024.100928
Prolonged use of nomegestrol acetate and risk of intracranial meningioma: a population-based cohort study
Abstract
Background: Nomegestrol acetate (NOMAC) is a synthetic potent progestogen. This study aimed to assess the risk of intracranial meningioma associated with the prolonged use of NOMAC.
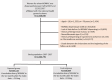
Methods: Observational cohort study using SNDS data (France). Women included had ≥ one dispensing of NOMAC between 2007 and 2017 (no dispensing in 2006). Exposure was defined as a cumulative dose >150 mg NOMAC within six months after first dispensing. A control group of women (cumulative dose ≤150 mg) was assembled. The outcome was surgery (resection or decompression) or radiotherapy for one or more intracranial meningioma(s). Poisson models assessed the relative risk (RR) of meningioma.
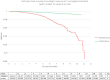
Findings: In total, 1,060,779 women were included in the cohort (535,115 in the exposed group and 525,664 in the control group). The incidence of meningioma in the two groups was 19.3 and 7.0 per 100,000 person-years, respectively (age-adjusted RRa = 2.9 [2.4-3.7]). The RRa for a cumulative dose of more than 6 g NOMAC was 12.0 [9.9-16.0]. In the event of treatment discontinuation for at least one year, the risk of meningioma was identical to that in the control group (RRa = 1.0 [0.8-1.3]). The location of meningiomas in the anterior and middle part of the skull base was more frequent with exposure to NOMAC.
Interpretation: We observed a strong dose-dependent association between prolonged use of NOMAC and the risk of intracranial meningiomas. These results are comparable to those obtained for cyproterone acetate, although the magnitude of the risk is lower. It is now recommended to stop using NOMAC if a meningioma is diagnosed.
Funding: The French National Health Insurance Fund (Cnam) and the French National Agency for Medicines and Health Products Safety (ANSM) via the Health Product Epidemiology Scientific Interest Group EPI-PHARE.
Keywords: Meningioma; Nomegestrol acetate; Pharmacoepidemiology; Progestin.
© 2024 The Author(s).
Conflict of interest statement
We declare no financial relationships with any organizations that might have an interest in the submitted work in the previous three years and no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
-
- Lello S. Nomegestrol acetate: pharmacology, safety profile and therapeutic efficacy. Drugs. 2010;70(5):541–559. - PubMed
-
- Chakravarthy V., Kaplan B., Gospodarev V., Myers H., De Los Reyes K., Achiriloaie A. Houdini tumor: case report and literature review of pregnancy-associated meningioma. World Neurosurg. 2018;114:e1261–e1265. - PubMed
LinkOut - more resources
Full Text Sources

