A Randomized Controlled Trial Comparing Efficacy and Safety of Antidepressant Monotherapy
- PMID: 38800340
- PMCID: PMC11128267
- DOI: 10.7759/cureus.59074
A Randomized Controlled Trial Comparing Efficacy and Safety of Antidepressant Monotherapy
Abstract
Background and objectives: The majority of mainstream antidepressants lack the promise of complete amelioration of symptoms. Other pitfalls include the latency period and side effects. These issues prompted investigations concerning the various roles of serotonin (5-HT) neurotransmissions in the etiology of depression. In this study, each study participant received vilazodone, vortioxetine, and escitalopram monotherapy for major depressive disorder (MDD) for 16 weeks. After that, the subject's scores on the Hamilton Depression Rating Scale (HDRS)-17 item version and the Montgomery Åsberg Depression Rating Scale (MADRS) were evaluated. In the study population, we kept track of the incidence of adverse events.
Methods: Ninety-six patients with MDD participated in this open-label, randomized, three-arm study. Participants were allotted into three groups according to a 1:1:1 ratio and given vilazodone (20-40 mg/day), vortioxetine (5-20 mg/day), or escitalopram (10-20 mg/day) for 16 weeks. Vortioxetine and vilazodone are test medications, with escitalopram serving as the control. After the baseline visit, follow-up appointments were scheduled every four weeks. Per-protocol (PP) and intent-to-treat (ITT) populations served as means for efficacy and safety evaluations, respectively. We prospectively registered this research in the Clinical Trial Registry, India (CTRI) (2022/07/043808).
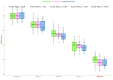
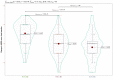
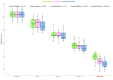
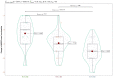
Results: Out of the 134 patients we screened, 109 (81.34%) were eligible. Ninety-six (88.07%) of them completed the 16-week trial. In the PP population (n = 96), we analyzed efficacy. They had a mean age of 46.3 ± 6.2 years. At baseline, each group's median HDRS score was 30.0 (p = 0.964). Following 16 weeks of antidepressant therapy, these scores dropped to 15.0, 14.0, and 13.0 (p = 0.002). Baseline MADRS scores for all groups were 36.0 (p = 0.741). They had corresponding values of 20.0, 18.0, and 17.0 at 16 weeks (p < 0.001). Regarding both efficacy endpoints, the post-hoc analysis with the Bonferroni correction demonstrated statistically significant differences (p < 0.001). We performed the safety assessments within our ITT population (n = 109). Ninety-six adverse events were recorded. Nonetheless, none of them seemed serious. Still, five participants opted out because of their side effects. Vomiting and nausea were the most frequent side effects.
Conclusion: Compared to escitalopram and vilazodone, vortioxetine demonstrated a statistically significant reduction in HDRS and MADRS scores. It also had fewer and milder side effects. We recommend conducting studies involving a broader population to investigate the antidepressant effects of these medications further.
Keywords: adverse event; antidepressant drug; depressive disorder; escitalopram; hamilton depression rating scale; montgomery-asberg depression rating scale; randomized trial; serotonin dysfunction; vilazodone; vortioxetine.
Copyright © 2024, Santi et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Finding intestinal fortitude: integrating the microbiome into a holistic view of depression mechanisms, treatment, and resilience. Flux MC, Lowry CA. https://doi.org/10.1016/j.nbd.2019.104578. Neurobiol Dis. 2020;135:104578. - PMC - PubMed
-
- Changes in the global burden of depression from 1990 to 2017: findings from the Global Burden of Disease study. Liu Q, He H, Yang J, Feng X, Zhao F, Lyu J. https://doi.org/10.1016/j.jpsychires.2019.08.002. J Psychiatr Res. 2020;126:134–140. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
