Survival after docetaxel for metastatic castration-resistant prostate cancer in a rural health care setting
- PMID: 38800536
- PMCID: PMC11117159
- DOI: 10.5114/wo.2024.138842
Survival after docetaxel for metastatic castration-resistant prostate cancer in a rural health care setting
Abstract
Introduction: The aim of this study was to evaluate overall survival of men who received systemic therapy with docetaxel for metastatic castration- resistant prostate cancer (MCRPC) in rural Nordland County, Norway. Prognostic factors related to treatment and other variables were evaluated.
Material and methods: Overall, 132 pa- tients were included in this retrospective study covering the years 2009-2022. Uni- and multivariate survival analyses were performed.
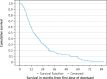
Results: In this elderly cohort (median age 72 years), weekly low-dose docetaxel was the preferred regimen (44%). Seventy-three percent were treated in the first line. Only 11 patients (8%) were pre-exposed to docetaxel in the hormone-sensitive phase. Median survival was 14.3 months. Prognostic factors for longer survival included higher hemoglobin, lower lactate dehydrogenase, administration of docetaxel as first-line MCRPC treatment, and use of fewer prescription drugs for comorbidity. Pre-exposure to docetaxel did not play a major role, p = 0.76.
Conclusions: In this rural health care setting, survival after docetaxel was shorter than reported by other groups. Blood test results were confirmed as important prognostic factors. In the present era of evolving treatment sequences, we recommend monitoring of real-world treatment results.
Keywords: chemotherapy; distant metastases; pattern of care; prostate cancer; survival; systemic therapy.
Copyright © 2024 Termedia.
Conflict of interest statement
None.
Figures
Similar articles
-
Feasibility and efficacy of early docetaxel plus androgen deprivation therapy for metastatic hormone-sensitive prostate cancer in a rural health care setting.Scand J Urol. 2022 Apr;56(2):114-118. doi: 10.1080/21681805.2022.2028006. Epub 2022 Feb 17. Scand J Urol. 2022. PMID: 35174779
-
Is there a seasonal variation of survival after systemic chemotherapy for metastatic castration-resistant prostate cancer in a rural part of North Norway?Int J Circumpolar Health. 2020 Dec;79(1):1742520. doi: 10.1080/22423982.2020.1742520. Int J Circumpolar Health. 2020. PMID: 32191614 Free PMC article.
-
Anticancer Activity and Tolerance of Treatments Received Beyond Progression in Men Treated Upfront with Androgen Deprivation Therapy With or Without Docetaxel for Metastatic Castration-naïve Prostate Cancer in the GETUG-AFU 15 Phase 3 Trial.Eur Urol. 2018 May;73(5):696-703. doi: 10.1016/j.eururo.2017.09.022. Epub 2017 Oct 23. Eur Urol. 2018. PMID: 29074061 Clinical Trial.
-
Docetaxel Activity in the Era of Life-prolonging Hormonal Therapies for Metastatic Castration-resistant Prostate Cancer.Eur Urol. 2016 Sep;70(3):410-2. doi: 10.1016/j.eururo.2016.05.002. Epub 2016 May 13. Eur Urol. 2016. PMID: 27184379 Review.
-
PSMA-targeted radioligand therapy in prostate cancer: current status and future prospects.Expert Rev Anticancer Ther. 2023 Jul-Dec;23(9):959-975. doi: 10.1080/14737140.2023.2247562. Epub 2023 Aug 28. Expert Rev Anticancer Ther. 2023. PMID: 37565281 Review.
Cited by
-
Impact of Body Mass Index on Survival After Docetaxel Chemotherapy for Metastatic Castration-resistant Prostate Cancer.Cancer Diagn Progn. 2025 Mar 3;5(2):138-145. doi: 10.21873/cdp.10423. eCollection 2025 Mar-Apr. Cancer Diagn Progn. 2025. PMID: 40034952 Free PMC article.
References
-
- Chi KN, Agarwal N, Bjartell A, et al. . TITAN investigators. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med 2019; 381: 13-24. - PubMed
LinkOut - more resources
Full Text Sources