Engineered CAR-T cells targeting the non-functional P2X purinoceptor 7 (P2X7) receptor as a novel treatment for ovarian cancer
- PMID: 38800555
- PMCID: PMC11116765
- DOI: 10.1002/cti2.1512
Engineered CAR-T cells targeting the non-functional P2X purinoceptor 7 (P2X7) receptor as a novel treatment for ovarian cancer
Abstract
Objectives: Recent studies have identified expression of the non-functional P2X7 (nfP2X7) receptor on various malignant cells including ovarian cancer, but not on normal cells, which makes it a promising tumour-associated antigen candidate for chimeric antigen receptor (CAR)-T-cell immunotherapies. In this study, we assessed the cytotoxic effects of nfP2X7-CAR-T cells on ovarian cancer using in vitro and in vivo models.
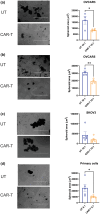
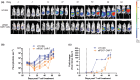
Methods: We evaluated the effects of nfP2X7-CAR-T cells on ovarian cancer cell lines (SKOV-3, OVCAR3, OVCAR5), normal peritoneal cells (LP-9) and primary serous ovarian cancer cells derived from patient ascites in vitro using monolayer and 3D spheroid assays. We also evaluated the effects of nfP2X7-CAR-T cells on patient-derived tissue explants, which recapitulate an intact tumour microenvironment. In addition, we investigated the effect of nfP2X7-CAR-T cells in vivo using the OVCAR-3 xenograft model in NOD-scid IL2Rγnull (NSG) mice.
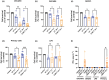
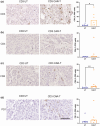
Results: Our study found that nfP2X7-CAR-T cells were cytotoxic and significantly inhibited survival of OVCAR3, OVCAR5 and primary serous ovarian cancer cells compared with un-transduced CD3+ T cells in vitro. However, no significant effects of nfP2X7-CAR-T cells were observed for SKOV3 or normal peritoneal cells (LP-9) cells with low P2X7 receptor expression. Treatment with nfP2X7-CAR-T cells increased apoptosis compared with un-transduced T cells in patient-derived explants and correlated with CD3 positivity. Treatment with nfP2X7-CAR-T cells significantly reduced OVCAR3 tumour burden in mice compared with un-transduced CD3 cells for 7-8 weeks.
Conclusion: This study demonstrates that nfP2X7-CAR-T cells have great potential to be developed as a novel immunotherapy for ovarian cancer.
Keywords: 3D‐spheroid; CAR‐T cells; explant assays; immunotherapy; in vivo; ovarian cancer.
© 2024 The Author(s). Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The authors report no conflict of interest.
Figures

References
-
- Sung H, Ferlay J, Siegel RL et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. - PubMed
-
- Colombo PE, Fabbro M, Theillet C, Bibeau F, Rouanet P, Ray‐Coquard I. Sensitivity and resistance to treatment in the primary management of epithelial ovarian cancer. Crit Rev Oncol Hematol 2014; 89: 207–216. - PubMed
LinkOut - more resources
Full Text Sources
