Polylactic acid micro/nanoplastic-induced hepatotoxicity: Investigating food and air sources via multi-omics
- PMID: 38800715
- PMCID: PMC11127520
- DOI: 10.1016/j.ese.2024.100428
Polylactic acid micro/nanoplastic-induced hepatotoxicity: Investigating food and air sources via multi-omics
Abstract
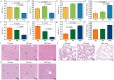
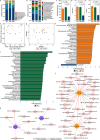
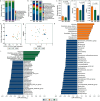
Micro/nanoplastics (MNPs) are detected in human liver, and pose significant risks to human health. Oral exposure to MNPs derived from non-biodegradable plastics can induce toxicity in mouse liver. Similarly, nasal exposure to non-biodegradable plastics can cause airway dysbiosis in mice. However, the hepatotoxicity induced by foodborne and airborne biodegradable MNPs remains poorly understood. Here we show the hepatotoxic effects of biodegradable polylactic acid (PLA) MNPs through multi-omics analysis of various biological samples from mice, including gut, fecal, nasal, lung, liver, and blood samples. Our results show that both foodborne and airborne PLA MNPs compromise liver function, disrupt serum antioxidant activity, and cause liver pathology. Specifically, foodborne MNPs lead to gut microbial dysbiosis, metabolic alterations in the gut and serum, and liver transcriptomic changes. Airborne MNPs affect nasal and lung microbiota, alter lung and serum metabolites, and disrupt liver transcriptomics. The gut Lachnospiraceae_NK4A136_group is a potential biomarker for foodborne PLA MNP exposure, while nasal unclassified_Muribaculaceae and lung Klebsiella are potential biomarkers for airborne PLA MNP exposure. The relevant results suggest that foodborne PLA MNPs could affect the "gut microbiota-gut-liver" axis and induce hepatoxicity, while airborne PLA MNPs could disrupt the "airway microbiota-lung-liver" axis and cause hepatoxicity. These findings have implications for diagnosing PLA MNPs-induced hepatotoxicity and managing biodegradable materials in the environment. Our current study could be a starting point for biodegradable MNPs-induced hepatotoxicity. More research is needed to verify and inhibit the pathways that are crucial to MNPs-induced hepatotoxicity.
Keywords: Hepatotoxicity; Metabolic disruption; Micro/nanoplastics; Microbiota dysbiosis; Transcriptomic dysregulation.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Bagheri T., Gholizadeh M., Abarghouei S., Zakeri M., Hedayati A., Rabaniha M., Aghaeimoghadam A., Hafezieh M. Microplastics distribution, abundance and composition in sediment, fishes and benthic organisms of the Gorgan Bay, Caspian sea. Chemosphere. 2020;257 - PubMed
-
- Banaei G., García-Rodríguez A., Tavakolpournegari A., Martín-Pérez J., Villacorta A., Marcos R., Hernández A. The release of polylactic acid nanoplastics (PLA-NPLs) from commercial teabags. Obtention, characterization, and hazard effects of true-to-life PLA-NPLs. J. Hazard. Mater. 2023 - PubMed
-
- Senathirajah K., Attwood S., Bhagwat G., Carbery M., Wilson S., Palanisami T. Estimation of the mass of microplastics ingested–A pivotal first step towards human health risk assessment. J. Hazard. Mater. 2021;404 - PubMed
LinkOut - more resources
Full Text Sources

