My Vision of Electric-Field-Aided Chemistry in 2050
- PMID: 38800723
- PMCID: PMC11117677
- DOI: 10.1021/acsphyschemau.3c00064
My Vision of Electric-Field-Aided Chemistry in 2050
Abstract
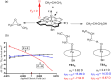
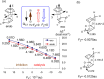
This manuscript outlines my outlook on the development of electric-field (EF)-mediated-chemistry and the vision of its state by 2050. I discuss applications of oriented-external electric-fields (OEEFs) on chemical reactions and proceed with relevant experimental verifications. Subsequently, the Perspective outlines other ways of generating EFs, e.g., by use of pH-switchable charges, ionic additives, water droplets, and so on. A special section summarizes conceptual principles for understanding and predicting OEEF effects, e.g., the "reaction-axis rule", the capability of OEEFs to act as tweezers that orient reactants and accelerate their reaction, etc. Finally, I discuss applications of OEEFs in continuous-flow setups, which may, in principle, scale-up to molar concentrations. The Perspective ends with the vision that by 2050, OEEF usage will change chemical education, if not also the art of making new molecules.
© 2024 The Author. Published by American Chemical Society.
Conflict of interest statement
The author declares no competing financial interest.
Figures







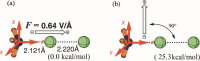



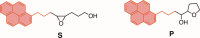
References
-
- Effects of Electric Fields on Structure and Reactivity: New Horizons in Chemistry ;Shaik S., Stuyver T., Eds.; Royal Society of Chemistry, 2021; pp 1–428.10.1039/9781839163043. - DOI
-
- Shaik S.; Danovich D.; Dubey K. D.; Stuyver T.. The Impact of Electric Fields on Chemical Structure and Reactivity. In Effects of Electric Fields on Structure and Reactivity: New Horizons in Chemistry ;Royal Society of Chemistry, 2021; pp12–70. 10.1039/9781839163043-00012. - DOI
Publication types
LinkOut - more resources
Full Text Sources
