Raman Spectroscopic Insights of Phase-Separated Insulin Aggregates
- PMID: 38800728
- PMCID: PMC11117687
- DOI: 10.1021/acsphyschemau.3c00065
Raman Spectroscopic Insights of Phase-Separated Insulin Aggregates
Abstract
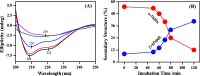
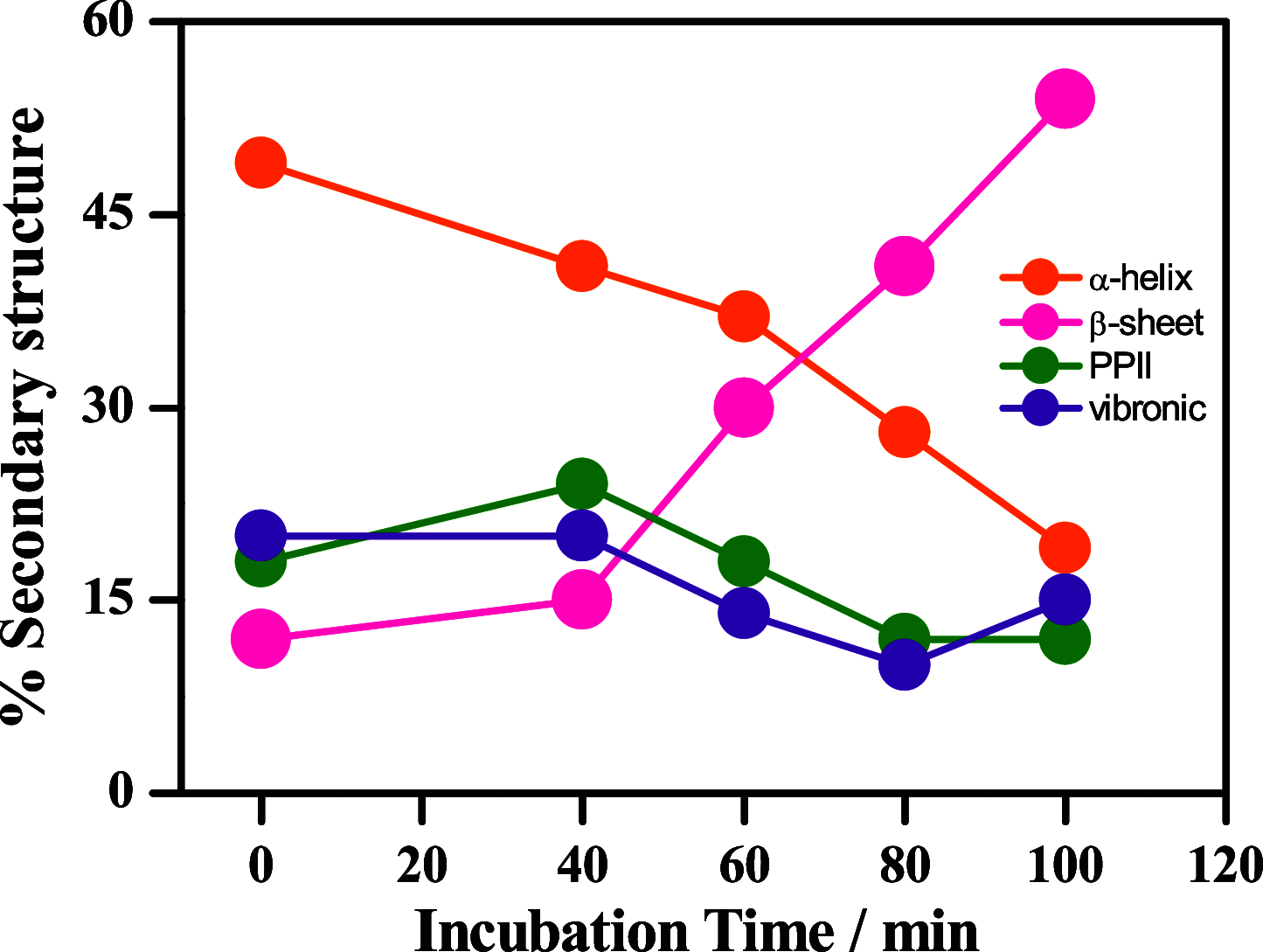
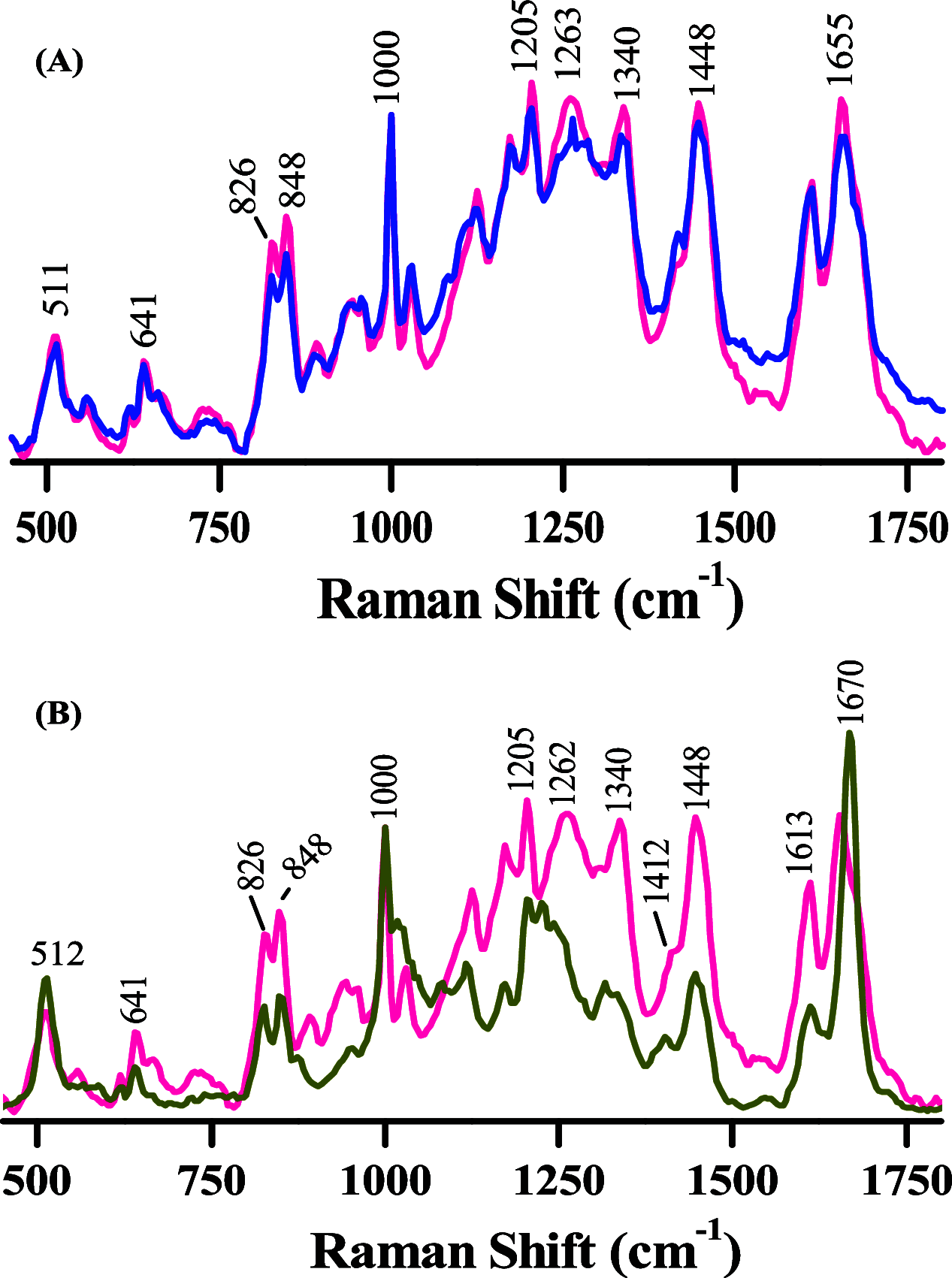
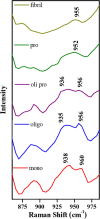
Phase-separated protein accumulation through the formation of several aggregate species is linked to the pathology of several human disorders and diseases. Our current investigation envisaged detailed Raman signature and structural intricacy of bovine insulin in its various forms of aggregates produced in situ at an elevated temperature (60 °C). The amide I band in the Raman spectrum of the protein in its native-like conformation appeared at 1655 cm-1 and indicated the presence of a high content of α-helical structure as prepared freshly in acidic pH. The disorder content (turn and coils) also was predominately present in both the monomeric and oligomeric states and was confirmed by the presence shoulder amide I maker band at ∼1680 cm-1. However, the band shifted to ∼1671 cm-1 upon the transformation of the protein solution into fibrillar aggregates as produced for a longer time of incubation. The protein, however, maintained most of its helical conformation in the oligomeric phase; the low-frequency backbone α-helical conformation signal at ∼935 cm-1 was similar to that of freshly prepared aqueous protein solution enriched in helical conformation. The peak intensity was significantly weak in the fibrillar aggregates, and it appeared as a good Raman signature to follow the phase separation and the aggregation behavior of insulin and similar other proteins. Tyrosine phenoxy moieties in the protein may maintained its H-bond donor-acceptor integrity throughout the course of fibril formation; however, it entered in more hydrophobic environment in its journey of fibril formation. In addition, it was noticed that oligomeric bovine insulin maintained the orientation/conformation of the disulfide bonds. However, in the fibrillar state, the disulfide linkages became more strained and preferred to maintain a single conformation state.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Carulla N.; Zhou M.; Arimon M.; Gairí M.; Giralt E.; Robinson C. V.; Dobson C. M. Experimental Characterization of Disordered and Ordered Aggregates Populated during the Process of Amyloid Fibril Formation. Proc. Natl. Acad. Sci. U. S. A. 2009, 106 (19), 7828–7833. 10.1073/pnas.0812227106. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
