Immune checkpoint inhibitors: breakthroughs in cancer treatment
- PMID: 38801082
- PMCID: PMC11208906
- DOI: 10.20892/j.issn.2095-3941.2024.0055
Immune checkpoint inhibitors: breakthroughs in cancer treatment
Abstract
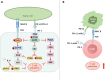
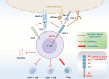
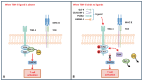
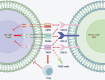
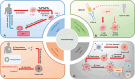
Over the past two decades, immunotherapies have increasingly been considered as first-line treatments for most cancers. One such treatment is immune checkpoint blockade (ICB), which has demonstrated promising results against various solid tumors in clinical trials. Monoclonal antibodies (mAbs) are currently available as immune checkpoint inhibitors (ICIs). These ICIs target specific immune checkpoints, including cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) and programmed cell death protein 1 (PD-1). Clinical trial results strongly support the feasibility of this immunotherapeutic approach. However, a substantial proportion of patients with cancer develop resistance or tolerance to treatment, owing to tumor immune evasion mechanisms that counteract the host immune response. Consequently, substantial research focus has been aimed at identifying additional ICIs or synergistic inhibitory receptors to enhance the effectiveness of anti-PD-1, anti-programmed cell death ligand 1 (anti-PD-L1), and anti-CTLA-4 treatments. Recently, several immune checkpoint molecular targets have been identified, such as T cell immunoreceptor with Ig and ITIM domains (TIGIT), mucin domain containing-3 (TIM-3), lymphocyte activation gene-3 (LAG-3), V-domain immunoglobulin suppressor of T cell activation (VISTA), B and T lymphocyte attenuator (BTLA), and signal-regulatory protein α (SIRPα). Functional mAbs targeting these molecules are under development. CTLA-4, PD-1/PD-L1, and other recently discovered immune checkpoint proteins with distinct structures are at the forefront of research. This review discusses these structures, as well as clinical progress in mAbs targeting these immune checkpoint molecules and their potential applications.
Keywords: CTLA-4; ICIs; Immunotherapy; PD-1; cancer.
Copyright: © 2024, The Authors.
Conflict of interest statement
No potential conflicts of interest are disclosed.
Figures


References
-
- Kang C. Retifanlimab: first approval. Drugs. 2023;83:731–7. - PubMed
-
- Gaikwad S, Agrawal MY, Kaushik I, Ramachandran S, Srivastava SK. Immune checkpoint proteins: signaling mechanisms and molecular interactions in cancer immunotherapy. Semin Cancer Biol. 2022;86:137–50. - PubMed
-
- Ljunggren H-G, Jonsson R, Höglund P. Seminal immunologic discoveries with direct clinical implications: The 2018 Nobel Prize in Physiology or Medicine honours discoveries in cancer immunotherapy. Scand J Immunol. 2018;88:e12731. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
