Lithium prevents glucocorticoid-induced osteonecrosis of the femoral head by regulating autophagy
- PMID: 38801405
- PMCID: PMC11129728
- DOI: 10.1111/jcmm.18385
Lithium prevents glucocorticoid-induced osteonecrosis of the femoral head by regulating autophagy
Abstract
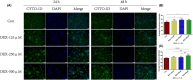
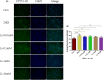
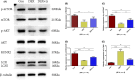
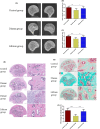
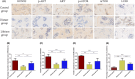
Autophagy may play an important role in the occurrence and development of glucocorticoid-induced osteonecrosis of the femoral head (GC-ONFH). Lithium is a classical autophagy regulator, and lithium can also activate osteogenic pathways, making it a highly promising therapeutic agent for GC-ONFH. We aimed to evaluate the potential therapeutic effect of lithium on GC-ONFH. For in vitro experiments, primary osteoblasts of rats were used for investigating the underlying mechanism of lithium's protective effect on GC-induced autophagy levels and osteogenic activity dysfunction. For in vivo experiments, a rat model of GC-ONFH was used for evaluating the therapeutic effect of oral lithium on GC-ONFH and underlying mechanism. Findings demonstrated that GC over-activated the autophagy of osteoblasts and reduced their osteogenic activity. Lithium reduced the over-activated autophagy of GC-treated osteoblasts through PI3K/AKT/mTOR signalling pathway and increased their osteogenic activity. Oral lithium reduced the osteonecrosis rates in a rat model of GC-ONFH, and restrained the increased expression of autophagy related proteins in bone tissues through PI3K/AKT/mTOR signalling pathway. In conclusion, lithium can restrain over-activated autophagy by activating PI3K/AKT/mTOR signalling pathway and up-regulate the expression of genes for bone formation both in GC induced osteoblasts and in a rat model of GC-ONFH. Lithium may be a promising therapeutic agent for GC-ONFH. However, the role of autophagy in the pathogenesis of GC-ONFH remains controversial. Studies are still needed to further explore the role of autophagy in the pathogenesis of GC-ONFH, and the efficacy of lithium in the treatment of GC-ONFH and its underlying mechanisms.
Keywords: autophagy; glucocorticoid; lithium; osteoblast; osteonecrosis of the femoral head.
© 2024 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Luo P, Gao F, Han J, Sun W, Li Z. The role of autophagy in steroid necrosis of the femoral head: a comprehensive research review. Int Orthop. 2018;42(7):1747‐1753. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

