Oxidative stress and NF-KB/iNOS inflammatory pathway as innovative biomarkers for diagnosis of drowning and differentiating it from postmortem submersion in both fresh and saltwater in rats
- PMID: 38801418
- PMCID: PMC11306576
- DOI: 10.1007/s00414-024-03249-5
Oxidative stress and NF-KB/iNOS inflammatory pathway as innovative biomarkers for diagnosis of drowning and differentiating it from postmortem submersion in both fresh and saltwater in rats
Abstract
Background: Finding a dead body in water raises an issue concerning determining the cause of death as drowning because of the complex pathophysiology of drowning. In addition, the corpse may be submersed postmortem.
Objective: Evaluate the role of oxidative stress markers and NF-KB/iNOS inflammatory pathway as diagnostic biomarkers in drowning and whether they could differentiate freshwater from saltwater drowning.
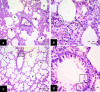
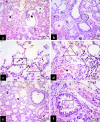
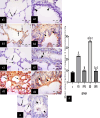
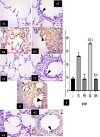
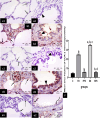
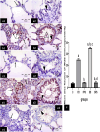
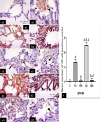
Methods: This study included forty-five adult male albino rats classified into five groups: control group (C), Freshwater-drowned group (FD), Freshwater postmortem submersion group (FPS), saltwater-drowned group (SD), and saltwater postmortem submersion group (SPS). After the autopsy, the rats' lungs in each group were prepared for histological, immunohistochemical (caspase 3, TNF-α, NF-kB, COX-2 & iNOS), biochemical studies; MDA, NOx, SOD, GSH, VCAM-1, COX-2; and RT-PCR for the relative quantification of NF-kB and iNOS genes expression.
Results: Lung oxidative markers were significantly affected in drowned groups than in postmortem submersion groups. Inflammatory pathway markers were also significantly increased in the drowned groups, with concern that all markers were significantly affected more in saltwater than in freshwater drowned group.
Conclusions: It is concluded that the tested markers can be used accurately in diagnosing drowning and differentiating it from postmortem submersion with a better understanding of the mechanism of death in drowning as both mechanisms, inflammatory and oxidative stress, were revealed and involved.
Keywords: Acute lung injury; Drowning; Freshwater; Postmortem submersion; Saltwater.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that there is no competing interest.
Figures

References
-
- Lee SY, Ha EJ, Cho HW, Kim HR, Lee D, Eom YB (2019) Potential forensic application of receptor for advanced glycation end products (RAGE) and aquaporin 5 (AQP5) as novel biomarkers for diagnosis of drowning. J Forensic Leg Med 62:56–62 - PubMed
-
- Girela-López E, Beltran-Aroca CM, Dye A, Gill JR (2022) Epidemiology and autopsy findings of 500 drowning deaths. Forensic Sci Int 330:111137 - PubMed
-
- Szpilman D, Morgan PJ (2021) Management for the drowning patient. Chest 159:1473–1483 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

