Is CAA a perivascular brain clearance disease? A discussion of the evidence to date and outlook for future studies
- PMID: 38801464
- PMCID: PMC11130115
- DOI: 10.1007/s00018-024-05277-1
Is CAA a perivascular brain clearance disease? A discussion of the evidence to date and outlook for future studies
Abstract
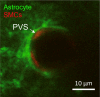
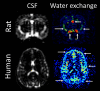
The brain's network of perivascular channels for clearance of excess fluids and waste plays a critical role in the pathogenesis of several neurodegenerative diseases including cerebral amyloid angiopathy (CAA). CAA is the main cause of hemorrhagic stroke in the elderly, the most common vascular comorbidity in Alzheimer's disease and also implicated in adverse events related to anti-amyloid immunotherapy. Remarkably, the mechanisms governing perivascular clearance of soluble amyloid β-a key culprit in CAA-from the brain to draining lymphatics and systemic circulation remains poorly understood. This knowledge gap is critically important to bridge for understanding the pathophysiology of CAA and accelerate development of targeted therapeutics. The authors of this review recently converged their diverse expertise in the field of perivascular physiology to specifically address this problem within the framework of a Leducq Foundation Transatlantic Network of Excellence on Brain Clearance. This review discusses the overarching goal of the consortium and explores the evidence supporting or refuting the role of impaired perivascular clearance in the pathophysiology of CAA with a focus on translating observations from rodents to humans. We also discuss the anatomical features of perivascular channels as well as the biophysical characteristics of fluid and solute transport.
Keywords: Brain clearance; Cerebral amyloid angiopathy; Cerebrospinal fluid; Glymphatics; IPAD; Perivascular spaces.
© 2024. The Author(s).
Conflict of interest statement
Authors E.B., H.B., R.C., S.L., G.P., and A.S., declare they have no financial interests. Author S.G. has served as a consultant for Eli Lily, as a safety monitoring committee member for Bayer and IQVIA/Washington University, and as a scientific advisory board member for MIAC, and has received industry support in the form of a sponsored research agreement from Alnylam. Author J.I. serves as the Chair for the Scientific Advisory Board of Applied Cognition, Inc. He received compensation for this work and holds an equity stake in this company. Author M.v.O has received industry support from Philips and serves as a consultant for Alnylam. Author W.V.N. has received industry support from Alnylam. Author S.v.V. has received industry support in the form of a sponsored research agreement from Therini Bio and Sanofi and has served as a consultant for Biogen and Eisai.
Figures




References
-
- Wardlaw JM, Benveniste H, Nedergaard M, Zlokovic BV, Mestre H, Lee H, Doubal FN, Brown R, Ramirez J, MacIntosh BJ, Tannenbaum A, Ballerini L, Rungta RL, Boido D, Sweeney M, Montagne A, Charpak S, Joutel A, Smith KJ, Black SE (2020) Perivascular spaces in the brain: anatomy, physiology and pathology. Nat Rev Neurol 16:137–153. 10.1038/s41582-020-0312-z - DOI - PubMed
-
- Koemans EA, Chhatwal JP, van Veluw SJ, van Etten ES, van Osch MJP, van Walderveen MAA, Sohrabi HR, Kozberg MG, Shirzadi Z, Terwindt GM, van Buchem MA, Smith EE, Werring DJ, Martins RN, Wermer MJH, Greenberg SM (2023) Progression of cerebral amyloid angiopathy: a pathophysiological framework. Lancet Neurol 22:632–642. 10.1016/S1474-4422(23)00114-X - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

