Neuropathological findings in Down syndrome, Alzheimer's disease and control patients with and without SARS-COV-2: preliminary findings
- PMID: 38801558
- PMCID: PMC11130011
- DOI: 10.1007/s00401-024-02743-9
Neuropathological findings in Down syndrome, Alzheimer's disease and control patients with and without SARS-COV-2: preliminary findings
Abstract
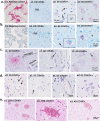
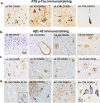
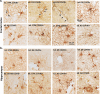
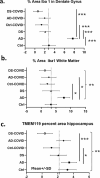
The SARS-CoV-2 virus that led to COVID-19 is associated with significant and long-lasting neurologic symptoms in many patients, with an increased mortality risk for people with Alzheimer's disease (AD) and/or Down syndrome (DS). However, few studies have evaluated the neuropathological and inflammatory sequelae in postmortem brain tissue obtained from AD and people with DS with severe SARS-CoV-2 infections. We examined tau, beta-amyloid (Aβ), inflammatory markers and SARS-CoV-2 nucleoprotein in DS, AD, and healthy non-demented controls with COVID-19 and compared with non-infected brain tissue from each disease group (total n = 24). A nested ANOVA was used to determine regional effects of the COVID-19 infection on arborization of astrocytes (Sholl analysis) and percent-stained area of Iba-1 and TMEM 119. SARS-CoV-2 antibodies labeled neurons and glial cells in the frontal cortex of all subjects with COVID-19, and in the hippocampus of two of the three DS COVID-19 cases. SARS-CoV-2-related alterations were observed in peri-vascular astrocytes and microglial cells in the gray matter of the frontal cortex, hippocampus, and para-hippocampal gyrus. Bright field microscopy revealed scattered intracellular and diffuse extracellular Aβ deposits in the hippocampus of controls with confirmed SARS-CoV-2 infections. Overall, the present preliminary findings suggest that SARS-CoV-2 infections induce abnormal inflammatory responses in Down syndrome.
Keywords: Alzheimer’s disease; Brain; COVID-19; Corona viruses; Down syndrome; Glial cells; Neurologic symptoms; SARS-CoV-2.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- R01AG071228-02/National Institutes of Aging
- R01AG070153/National Institutes of Aging
- R01 AG010668/AG/NIA NIH HHS/United States
- R01AG061566/National Institutes of Aging
- RF1 AG061566/AG/NIA NIH HHS/United States
- 20221216/Hans-Gabriel och Alice Trolle-Wachtmeisters stiftelse för medicinsk forskning
- P30AG066519/GF/NIH HHS/United States
- P01 AG014449/AG/NIA NIH HHS/United States
- AARG-22-974669/ALZ/Alzheimer's Association/United States
- RF1 AG081286/AG/NIA NIH HHS/United States
- RF1AG081286/GF/NIH HHS/United States
- CA2018010/BrightFocus Foundation
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

