Multimodal Imaging-Guided Stem Cell Ocular Treatment
- PMID: 38801653
- PMCID: PMC11804834
- DOI: 10.1021/acsnano.3c10632
Multimodal Imaging-Guided Stem Cell Ocular Treatment
Abstract
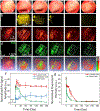
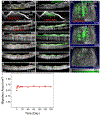
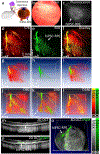
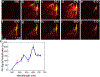
Stem cell therapies are gaining traction as promising treatments for a variety of degenerative conditions. Both clinical and preclinical studies of regenerative medicine are hampered by the lack of technologies that can evaluate the migration and behavior of stem cells post-transplantation. This study proposes an innovative method to longitudinally image in vivo human-induced pluripotent stem cells differentiated to retinal pigment epithelium (hiPSC-RPE) cells by multimodal photoacoustic microscopy, optical coherence tomography, and fluorescence imaging powered by ultraminiature chain-like gold nanoparticle cluster (GNC) nanosensors. The GNC exhibits an optical absorption peak in the near-infrared regime, and the 7-8 nm size in diameter after disassembly enables renal excretion and improved safety as well as biocompatibility. In a clinically relevant rabbit model, GNC-labeled hiPSC-RPE cells migrated to RPE degeneration areas and regenerated damaged tissues. The hiPSC-RPE cells' distribution and migration were noninvasively, longitudinally monitored for 6 months with exceptional sensitivity and spatial resolution. This advanced platform for cellular imaging has the potential to enhance regenerative cell-based therapies.
Keywords: contrast agents; gold nanochains; human-induced pluripotent stem cells differentiated to retinal pigment epithelium; optical coherence tomography; photoacoustic microscopy; regenerative medicine; stem cell therapy.
Conflict of interest statement
Competing interests
The authors declare no competing financial interests.
Figures






References
-
- Jonas JB; Cheung CMG; Panda-Jonas S, Updates on the epidemiology of age-related macular degeneration. The Asia-Pacific Journal of Ophthalmology 2017, 6 (6), 493–497; - PubMed
- Ding X; Patel M; Chan C-C, Molecular pathology of age-related macular degeneration. Prog. Retin. Eye Res. 2009, 28 (1), 1–18. - PMC - PubMed
-
- DeWan A; Liu M; Hartman S; Zhang SS-M; Liu DT; Zhao C; Tam PO; Chan WM; Lam DS; Snyder M, HTRA1 promoter polymorphism in wet age-related macular degeneration. Science 2006, 314 (5801), 989–992; - PubMed
- Stewart MW, Clinical and differential utility of VEGF inhibitors in wet age-related macular degeneration: focus on aflibercept. Clinical ophthalmology 2012, 1175–1186. - PMC - PubMed
-
- Rickman CB; Farsiu S; Toth CA; Klingeborn M, Dry age-related macular degeneration: mechanisms, therapeutic targets, and imaging. Invest. Ophthalmol. Vis. Sci. 2013, 54 (14), ORSF68-ORSF80; - PMC - PubMed
- de Guimaraes TAC; Varela MD; Georgiou M; Michaelides M, Treatments for dry age-related macular degeneration: therapeutic avenues, clinical trials and future directions. Br. J. Ophthalmol. 2022, 106 (3), 297–304. - PMC - PubMed
-
- Holz FG; Strauss EC; Schmitz-Valckenberg S; van Lookeren Campagne M, Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology 2014, 121 (5), 1079–1091. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

