SARS-CoV-2 Vaccination in the First Year After Hematopoietic Cell Transplant or Chimeric Antigen Receptor T-Cell Therapy: A Prospective, Multicenter, Observational Study
- PMID: 38801746
- PMCID: PMC11327798
- DOI: 10.1093/cid/ciae291
SARS-CoV-2 Vaccination in the First Year After Hematopoietic Cell Transplant or Chimeric Antigen Receptor T-Cell Therapy: A Prospective, Multicenter, Observational Study
Abstract
Background: The optimal timing of vaccination with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines after cellular therapy is incompletely understood. The objectives of this study are to determine whether humoral and cellular responses after SARS-CoV-2 vaccination differ if initiated <4 months versus 4-12 months after cellular therapy.
Methods: We conducted a multicenter, prospective, observational study at 30 cancer centers in the United States. SARS-CoV-2 vaccination was administered as part of routine care. We obtained blood prior to and after vaccinations at up to 5 time points and tested for SARS-CoV-2 spike (anti-S) IgG in all participants and neutralizing antibodies for Wuhan D614G, Delta B.1.617.2, and Omicron B.1.1.529 strains, as well as SARS-CoV-2-specific T-cell receptors, in a subgroup.
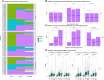
Results: We enrolled 466 allogeneic hematopoietic cell transplantation (HCT) (n = 231), autologous HCT (n = 170), and chimeric antigen receptor T-cell (CAR-T-cell) therapy (n = 65) recipients between April 2021 and June 2022. Humoral and cellular responses did not significantly differ among participants initiating vaccinations <4 months versus 4-12 months after cellular therapy. Anti-S IgG ≥2500 U/mL was correlated with high neutralizing antibody titers and attained by the last time point in 70%, 69%, and 34% of allogeneic HCT, autologous HCT, and CAR-T-cell recipients, respectively. SARS-CoV-2-specific T-cell responses were attained in 57%, 83%, and 58%, respectively. Pre-cellular therapy SARS-CoV-2 infection or vaccination and baseline B-cell count were key predictors of post-cellular therapy immunity.
Conclusions: These data support mRNA SARS-CoV-2 vaccination prior to, and reinitiation 3 to 4 months after, cellular therapies with allogeneic HCT, autologous HCT, and CAR-T-cell therapy.
Keywords: COVID-19; SARS-CoV-2; hematopoietic cell transplant; transplant; vaccine.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest . J. A. H—research funding: AlloVir, Geovax, Merck; consulting: Pfizer, Gilead, Moderna, Geovax, AlloVir. J. H. Y.—research funding: AlloVir, Ansun, Cidara, F2G, GSK, NobelPharma, Pulmocide, Scynexis, Shire/Takeda. L. W. L.—employment and equity holder in Adaptive Biotechnologies Corporation. M. V. D.—research funding: Janssen, Roche/Genentech. R. N.—consulting: Ono Pharmaceutical, Jazz Pharmaceuticals, Bluebird Bio, Omeros Pharmaceutical, Sanofi, Pfizer. J. M.—consulting: Evision, Kite, Allovir, Bristol Myers Squibb, Novartis, CRISPR, Nektar, Caribiou Bio, Sana Technologies, Legend Biotech. S. D.—research funding: MSK Leukemia SPORE Career Enhancement Program and MSK Gerstner Physician Scholar program. J. J. A.—employment: National Marrow Donor Program; Advisory Board: AscellaHealth, Takeda. M. H.—research support/funding: Takeda Pharmaceutical Company, ADC Therapeutics, Spectrum Pharmaceuticals, Astellas Pharma; consultancy: Incyte Corporation, MorphoSys, SeaGen, Gamida Cell, Novartis, Legend Biotech, Kadmon, ADC Therapeutics, Omeros, AbbVie, Caribou, CRISPR, Genmab, Kite; Speaker’s Bureau: Sanofi Genzyme, AstraZeneca, BeiGene, ADC Therapeutics, Kite; Data Monitoring Committee: Myeloid Therapeutics, Inc. M. L. R.—research funding from Jazz Pharmaceuticals and Atara Bio-Pharma; employment by IQVIA Biotech and Kura Oncology; stock in Kura Oncology. M. M. H.—research funding from Astellas Pharma, CSL Behring, Incyte, Sanofi; consultancy: Sobi, Inc. M.-A. P.—honoraria from Adicet, Allogene, Allovir, Caribou Biosciences, Celgene, Bristol-Myers Squibb, Equilium, Exevir, ImmPACT Bio, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Nektar Therapeutics, Novartis, Omeros, OrcaBio, Sanofi, Syncopation, VectivBio AG, and Vor Biopharma; he serves on Data and Safety Monitoring Boards (DSMBs) for Cidara Therapeutics and Sellas Life Sciences, and the scientific advisory board of NexImmune; he has ownership interests in NexImmune, Omeros, and OrcaBio; he has received institutional research support for clinical trials from Allogene, Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics, and Novartis. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures



Update of
-
SARS-CoV-2 vaccination in the first year after hematopoietic cell transplant or chimeric antigen receptor T cell therapy: A prospective, multicenter, observational study (BMT CTN 2101).medRxiv [Preprint]. 2024 Jan 25:2024.01.24.24301058. doi: 10.1101/2024.01.24.24301058. medRxiv. 2024. Update in: Clin Infect Dis. 2024 Aug 16;79(2):542-554. doi: 10.1093/cid/ciae291. PMID: 38343800 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
- Janssen/Johnson & Johnson
- OptumHealth
- Takeda Oncology Co
- Miltenyi Biotec, Inc
- Janssen Research & Development, LLC
- Xenikos BV
- Talaris Therapeutics
- Kyowa Kirin
- U10 HL069294/HL/NHLBI NIH HHS/United States
- Karyopharm Therapeutics
- Sanofi Genzyme
- MorphoSys
- U10HL069294/National Cancer Institute [NCI]
- Karius
- Vertex
- OncoImmune, Inc
- Orca Biosystems, Inc
- Kyowa Kirin International plc
- Seagen, Inc
- P30 CA015704/CA/NCI NIH HHS/United States
- Bristol Myers Squibb Co
- HistoGenetics
- P01 CA023766/CA/NCI NIH HHS/United States
- Millennium
- U24 CA076518/CA/NCI NIH HHS/United States
- Oncopeptides, Inc
- U24 HL138660/HL/NHLBI NIH HHS/United States
- AlloVir, Inc
- UG1 HL069315/HL/NHLBI NIH HHS/United States
- UG1 HL138645/HL/NHLBI NIH HHS/United States
- K08 CA293271/CA/NCI NIH HHS/United States
- HRSA/HRSA HHS/United States
- Terumo Blood and Cell Technologies
- N00014-20-1-2705/Department of Health and Human Services [DHHS]
- Adienne SA
- Novartis
- Kiadis Pharma
- UG1 HL108987/HL/NHLBI NIH HHS/United States
- Actinium Pharmaceuticals, Inc
- UG1 HL069278/HL/NHLBI NIH HHS/United States
- Bluebird Bio, Inc
- Novartis Pharmaceuticals Corporation
- Medical College of Wisconsin
- HHSH234200637015C/National Institute of Allergy and Infectious Diseases
- UG1 HL069246/HL/NHLBI NIH HHS/United States
- TG Therapeutics
- Pfizer, Inc
- Kite Pharma, Inc
- P30 CA008748/CA/NCI NIH HHS/United States
- Incyte Corporation
- Pharmacyclics, LLC
- Tscan
- National Heart, Lung, and Blood Institute [NHLBI]
- CytoSen Therapeutics, Inc
- Gilead
- Astellas Pharma US
- Takeda Pharmaceuticals
- Accenture
- AbbVie
- Gilead Company
- Be the Match Foundation
- Leukemia and Lymphoma Society
- Adaptive Biotechnologies
- National Marrow Donor Program/Be the Match
- Multiple Myeloma Research Foundation
- Stemcyte
- DBA Eurofins Transplant Diagnostics
- CareDx
- Eurofins Viracor
- NH/NIH HHS/United States
- CSL Behring
- Medac GmbH
- GlaxoSmithKline
- Fate Therapeutics
- American Society for Transplantation and Cellular Therapy
- Gamida-Cell, Ltd
- NCI
- Legend Biotech
- Kadmon
- Ossium Health, Inc
- Vor Biopharma
- Jasper Therapeutics
- Jazz Pharmaceuticals, Inc
- Iovance
- U24CA076518
- LabCorp
- Omeros Corporation
- Amgen, Inc
- Medexus, Merck & Co.
- Magenta Therapeutics
- Daiichi Sankyo Co, Ltd
- Priothera
- Office of Naval Research
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

