Does Adjunctive Clindamycin Have a Role in Staphylococcus aureus Bacteremia? A Protocol for the Adjunctive Treatment Domain of the Staphylococcus aureus Network Adaptive Platform (SNAP) Randomized Controlled Trial
- PMID: 38801783
- PMCID: PMC11426255
- DOI: 10.1093/cid/ciae289
Does Adjunctive Clindamycin Have a Role in Staphylococcus aureus Bacteremia? A Protocol for the Adjunctive Treatment Domain of the Staphylococcus aureus Network Adaptive Platform (SNAP) Randomized Controlled Trial
Abstract
Background: The use of adjunctive antibiotics directed against exotoxin production in Staphylococcus aureus bacteremia (SAB) is widespread, and it is recommended in many guidelines, but this is based on limited evidence. Existing guidelines are based on the theoretical premise of toxin suppression, as many strains of S. aureus produce toxins such as leukocidins (eg, Panton-Valentine leukocidin, toxic shock syndrome toxin 1, exfoliative toxins, and various enterotoxins). Many clinicians therefore believe that limiting exotoxin production release by S. aureus could reduce its virulence and improve clinical outcomes. Clindamycin, a protein synthesis inhibitor antibiotic, is commonly used for this purpose. We report the domain-specific protocol, embedded in a large adaptive, platform trial, seeking to definitively answer this question.
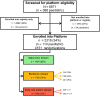
Methods and analysis: The Staphylococcus aureus Network Adaptive Platform (SNAP) trial is a pragmatic, randomized, multicenter adaptive platform trial that aims to compare different SAB therapies, simultaneously, for 90-day mortality rates. The adjunctive treatment domain aims to test the effectiveness of adjunctive antibiotics, initially comparing clindamycin to no adjunctive antibiotic, but future adaptations may include other agents. Individuals will be randomized to receive either 5 days of adjunctive clindamycin (or lincomycin) or no adjunctive antibiotic therapy alongside standard-of-care antibiotics. Most participants with SAB (within 72 hours of index blood culture and with no contraindications) will be eligible to participate in this domain. Prespecified analyses are defined in the statistical appendix to the core protocol, and domain-specific secondary analyses will be adjusted for resistance to clindamycin, disease phenotype (complicated or uncomplicated SAB) and Panton-Valentine leukocidin-positive isolate.
Keywords: Staphylococcus aureus bacteremia; adults; clindamycin; pediatrics; randomized controlled trial.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

