Hexokinase 2 nonmetabolic function-mediated phosphorylation of IκBα enhances pancreatic ductal adenocarcinoma progression
- PMID: 38801832
- PMCID: PMC11309947
- DOI: 10.1111/cas.16204
Hexokinase 2 nonmetabolic function-mediated phosphorylation of IκBα enhances pancreatic ductal adenocarcinoma progression
Abstract
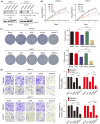
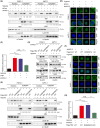
Aberrant signaling in tumor cells induces nonmetabolic functions of some metabolic enzymes in many cellular activities. As a key glycolytic enzyme, the nonmetabolic function of hexokinase 2 (HK2) plays a role in tumor immune evasion. However, whether HK2, dependent of its nonmetabolic activity, plays a role in human pancreatic ductal adenocarcinoma (PDAC) tumorigenesis remains unclear. Here, we demonstrated that HK2 acts as a protein kinase and phosphorylates IκBα at T291 in PDAC cells, activating NF-κB, which enters the nucleus and promotes the expression of downstream targets under hypoxia. HK2 nonmetabolic activity-promoted activation of NF-κB promotes the proliferation, migration, and invasion of PDAC cells. These findings provide new insights into the multifaceted roles of HK2 in tumor development and underscore the potential of targeting HK2 protein kinase activity for PDAC treatment.
Keywords: HK2; IκBα; nonmetabolic activity; pancreatic ductal adenocarcinoma; tumor progression.
© 2024 The Author(s). Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
MIF/NR3C2 axis regulates glucose metabolism reprogramming in pancreatic cancer through MAPK-ERK and AP-1 pathways.Carcinogenesis. 2024 Aug 12;45(8):582-594. doi: 10.1093/carcin/bgae025. Carcinogenesis. 2024. PMID: 38629149 Free PMC article.
-
Glycolytic enzyme HK2 promotes PD-L1 expression and breast cancer cell immune evasion.Front Immunol. 2023 Jun 12;14:1189953. doi: 10.3389/fimmu.2023.1189953. eCollection 2023. Front Immunol. 2023. PMID: 37377974 Free PMC article.
-
lncRNA-PLACT1 sustains activation of NF-κB pathway through a positive feedback loop with IκBα/E2F1 axis in pancreatic cancer.Mol Cancer. 2020 Feb 21;19(1):35. doi: 10.1186/s12943-020-01153-1. Mol Cancer. 2020. PMID: 32085715 Free PMC article.
-
Advancement of NF-κB Signaling Pathway: A Novel Target in Pancreatic Cancer.Int J Mol Sci. 2018 Dec 5;19(12):3890. doi: 10.3390/ijms19123890. Int J Mol Sci. 2018. PMID: 30563089 Free PMC article. Review.
-
Metabolic plasticity in pancreatic cancer: The mitochondrial connection.Mol Metab. 2025 Feb;92:102089. doi: 10.1016/j.molmet.2024.102089. Epub 2024 Dec 28. Mol Metab. 2025. PMID: 39736443 Free PMC article. Review.
Cited by
-
Decoding microglial immunometabolism: a new frontier in Alzheimer's disease research.Mol Neurodegener. 2025 Mar 27;20(1):37. doi: 10.1186/s13024-025-00825-0. Mol Neurodegener. 2025. PMID: 40149001 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

