Effects of amyloid-β-mimicking peptide hydrogel matrix on neuronal progenitor cell phenotype
- PMID: 38801867
- PMCID: PMC11239292
- DOI: 10.1016/j.actbio.2024.05.020
Effects of amyloid-β-mimicking peptide hydrogel matrix on neuronal progenitor cell phenotype
Abstract
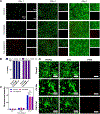
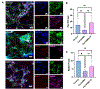
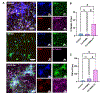
Self-assembling peptide-based hydrogels have become a highly attractive scaffold for three-dimensional (3D) in vitro disease modeling as they provide a way to create tunable matrices that can resemble the extracellular matrix (ECM) of various microenvironments. Alzheimer's disease (AD) is an exceptionally complex neurodegenerative condition; however, our understanding has advanced due to the transition from two-dimensional (2D) to 3D in vitro modeling. Nonetheless, there is a current gap in knowledge regarding the role of amyloid structures, and previously developed models found long-term difficulty in creating an appropriate model involving the ECM and amyloid aggregates. In this report, we propose a multi-component self-assembling peptide-based hydrogel scaffold to mimic the amyloid-beta (β) containing microenvironment. Characterization of the amyloid-β-mimicking hydrogel (Col-HAMA-FF) reveals the formation of β-sheet structures as a result of the self-assembling properties of phenylalanine (Phe, F) through π-π stacking of the residues, thus mimicking the amyloid-β protein nanostructures. We investigated the effect of the amyloid-β-mimicking microenvironment on healthy neuronal progenitor cells (NPCs) compared to a natural-mimicking matrix (Col-HAMA). Our results demonstrated higher levels of neuroinflammation and apoptosis markers when NPCs were cultured in the amyloid-like matrix compared to a natural brain matrix. Here, we provided insights into the impact of amyloid-like structures on NPC phenotypes and behaviors. This foundational work, before progressing to more complex plaque models, provides a promising scaffold for future investigations on AD mechanisms and drug testing. STATEMENT OF SIGNIFICANCE: In this study, we engineered two multi-component hydrogels: one to mimic the natural extracellular matrix (ECM) of the brain and one to resemble an amyloid-like microenvironment using a self-assembling peptide hydrogel. The self-assembling peptide mimics β-amyloid fibrils seen in amyloid-β protein aggregates. We report on the culture of neuronal progenitor cells within the amyloid-mimicking ECM scaffold to study the impact through marker expressions related to inflammation and DNA damage. This foundational work, before progressing to more complex plaque models, offers a promising scaffold for future investigations on AD mechanisms and drug testing.
Keywords: Alzheimer's disease; Amyloid fibrils; Hydrogel; Neuronal cells; Peptide self-assembly.
Copyright © 2024 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


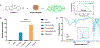
Similar articles
-
Polyethylene Glycol-Based Hydrogel as a 3D Extracellular Matrix Mimic for Cytotoxic T Lymphocytes.J Biomed Mater Res A. 2025 Jan;113(1):e37811. doi: 10.1002/jbm.a.37811. Epub 2024 Oct 21. J Biomed Mater Res A. 2025. PMID: 39429059
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Quantitative polarization microscopy as a potential tool for quantification of mechanical stresses within 3D matrices.Acta Biomater. 2025 Jun 15;200:236-250. doi: 10.1016/j.actbio.2025.04.052. Epub 2025 May 9. Acta Biomater. 2025. PMID: 40348695
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
Cited by
-
Nanotechnology in Drug Delivery: Anatomy and Molecular Insight into the Self-Assembly of Peptide-Based Hydrogels.Molecules. 2024 Nov 29;29(23):5654. doi: 10.3390/molecules29235654. Molecules. 2024. PMID: 39683812 Free PMC article. Review.
References
-
- Walsh DM, Selkoe DJ, Amyloid β-protein and beyond: The path forward in Alzheimer’s disease, Current opinion in neurobiology 61 (2020) 116–124. - PubMed
-
- Ashrafian H, Zadeh EH, Khan RH, Review on Alzheimer's disease: inhibition of amyloid beta and tau tangle formation, International journal of biological macromolecules 167 (2021) 382–394. - PubMed
-
- Gulisano W, Maugeri D, Baltrons MA, Fà M, Amato A, Palmeri A, D'Adamio L, Grassi C, Devanand D, Honig LS, Role of Amyloid-β and Tau Proteins in Alzheimer's Disease: Confuting the Amyloid Cascade, Journal of Alzheimer's Disease: JAD 68(1) (2019) 415–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

