Safety outcomes following COVID-19 vaccination and infection in 5.1 million children in England
- PMID: 38802362
- PMCID: PMC11130197
- DOI: 10.1038/s41467-024-47745-z
Safety outcomes following COVID-19 vaccination and infection in 5.1 million children in England
Erratum in
-
Author Correction: Safety outcomes following COVID-19 vaccination and infection in 5.1 million children in England.Nat Commun. 2024 Jul 8;15(1):5723. doi: 10.1038/s41467-024-50151-0. Nat Commun. 2024. PMID: 38977699 Free PMC article. No abstract available.
Abstract
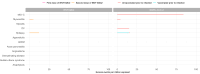
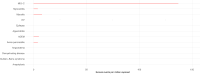
The risk-benefit profile of COVID-19 vaccination in children remains uncertain. A self-controlled case-series study was conducted using linked data of 5.1 million children in England to compare risks of hospitalisation from vaccine safety outcomes after COVID-19 vaccination and infection. In 5-11-year-olds, we found no increased risks of adverse events 1-42 days following vaccination with BNT162b2, mRNA-1273 or ChAdOX1. In 12-17-year-olds, we estimated 3 (95%CI 0-5) and 5 (95%CI 3-6) additional cases of myocarditis per million following a first and second dose with BNT162b2, respectively. An additional 12 (95%CI 0-23) hospitalisations with epilepsy and 4 (95%CI 0-6) with demyelinating disease (in females only, mainly optic neuritis) were estimated per million following a second dose with BNT162b2. SARS-CoV-2 infection was associated with increased risks of hospitalisation from seven outcomes including multisystem inflammatory syndrome and myocarditis, but these risks were largely absent in those vaccinated prior to infection. We report a favourable safety profile of COVID-19 vaccination in under-18s.
© 2024. The Author(s).
Conflict of interest statement
J.H.C. reports grants from National Institute for Health Research (NIHR) Biomedical Research Centre, Oxford, John Fell Oxford University Press Research Fund, Cancer Research UK and Oxford Wellcome Institutional Strategic Support Fund and other research councils, during the conduct of the study outside the scope of this work. J.H.C. is founder and was shareholder until 9 Aug 2023 of ClinRisk Ltd, which produces open and closed source software to implement clinical risk algorithms (outside this work) into clinical computer systems. J.H.C. is an unpaid director of QResearch, a not-for-profit organisation which is a partnership between the University of Oxford and EMIS Health who supply the QResearch database used for this work and is a consultant for Endeavour Predict Ltd outside this work. A.S. serves on a number of UK and Scottish Government COVID-19 advisory groups and was a member of AstraZeneca’s Thrombotic Thrombocytopenic Taskforce; all roles are unremunerated. A.H. is Deputy Chair of the Joint Committee on Vaccination and Immunisation. D.H. serves on the UK Government Commission on Human Medicines P.M. has received speaker and advisory board fees from Moderna and Astra Zeneca. All other authors declare no competing interests related to this paper.
Figures
References
-
- Health Policy Team. COVID-19 vaccination for children and young people. in Royal College of Paediatrics and Child Health, 2023 (2023).
-
- UK Health Security Agency. Week 19 report (up to week 18 data): 11 May 2023. in Weekly National Influenza and COVID-19 surveillance report (2023).
-
- UK Health Security Agency. COVID-19: the green book, chapter 14a. (2020).
-
- Department of Health and Social Care. JCVI statement on the COVID-19 vaccination programme for 2023: 8 November 2022. 2023 (2023).
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

