Dorsal raphe to basolateral amygdala corticotropin-releasing factor circuit regulates cocaine-memory reconsolidation
- PMID: 38802479
- PMCID: PMC11480471
- DOI: 10.1038/s41386-024-01892-5
Dorsal raphe to basolateral amygdala corticotropin-releasing factor circuit regulates cocaine-memory reconsolidation
Abstract
Environmental stimuli elicit drug craving and relapse in cocaine users by triggering the retrieval of strong cocaine-related contextual memories. Retrieval can also destabilize drug memories, requiring reconsolidation, a protein synthesis-dependent storage process, to maintain memory strength. Corticotropin-releasing factor (CRF) signaling in the basolateral amygdala (BLA) is necessary for cocaine-memory reconsolidation. We have hypothesized that a critical source of CRF in the BLA is the dorsal raphe nucleus (DR) based on its neurochemistry, anatomical connectivity, and requisite involvement in cocaine-memory reconsolidation. To test this hypothesis, male and female Sprague-Dawley rats received adeno-associated viruses to express Gi-coupled designer receptors exclusively activated by designer drugs (DREADDs) selectively in CRF neurons of the DR and injection cannulae directed at the BLA. The rats were trained to self-administer cocaine in a distinct environmental context then received extinction training in a different context. Next, they were briefly re-exposed to the cocaine-predictive context to destabilize (reactivate) cocaine memories. Intra-BLA infusions of the DREADD agonist deschloroclozapine (DCZ; 0.1 mM, 0.5 µL/hemisphere) immediately after memory reactivation attenuated cocaine-memory strength, relative to vehicle infusion. This was indicated by a selective, DCZ-induced and memory reactivation-dependent decrease in drug-seeking behavior in the cocaine-predictive context in DREADD-expressing males and females at test compared to respective controls. Notably, BLA-projecting DR CRF neurons that exhibited increased c-Fos expression during memory reconsolidation co-expressed the glutamatergic neuronal marker, vesicular glutamate transporter 3. Together, these findings suggest that the DRCRF → BLA circuit is engaged to maintain cocaine-memory strength after memory destabilization, and this phenomenon may be mediated by DR CRF and/or glutamate release in the BLA.
© 2024. The Author(s), under exclusive licence to American College of Neuropsychopharmacology.
Conflict of interest statement
The authors declare no competing interests.
Figures


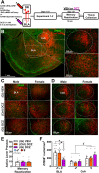
 sex main effect (underlined). All ps < 0.05.
sex main effect (underlined). All ps < 0.05.
 sex main effect; All p < 0.05. Aq cerebral aqueduct, dDR dorsal subregion of dorsal raphe, lDR lateral subregion of dorsal raphe, vDR ventral subregion of dorsal raphe, PAG periaqueductal gray.
sex main effect; All p < 0.05. Aq cerebral aqueduct, dDR dorsal subregion of dorsal raphe, lDR lateral subregion of dorsal raphe, vDR ventral subregion of dorsal raphe, PAG periaqueductal gray.Update of
-
Dorsal Raphe to Basolateral Amygdala Corticotropin-Releasing Factor Circuit Regulates Cocaine-Memory Reconsolidation.bioRxiv [Preprint]. 2024 Feb 12:2024.02.10.579725. doi: 10.1101/2024.02.10.579725. bioRxiv. 2024. Update in: Neuropsychopharmacology. 2024 Dec;49(13):2077-2086. doi: 10.1038/s41386-024-01892-5. PMID: 38405858 Free PMC article. Updated. Preprint.
References
-
- Bouton ME, Swartzentruber D. Analysis of the associative and occasion-setting properties of contexts participating in a Pavlovian discrimination. J Exp Psychol: Anim Beh Proc. 1986;12:333–50.
-
- O’brien CP, Childress AR, Mclellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Ann N Y Acad Sci. 1992;654:400–15. - PubMed
-
- Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–75. - PubMed
-
- Lee JL, Di Ciano P, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2005;47:795–801. - PubMed
-
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–84. - PubMed
MeSH terms
Substances
Grants and funding
- DA025646/U.S. Department of Health & Human Services | NIH | National Institute on Drug Abuse (NIDA)
- DA057330/U.S. Department of Health & Human Services | NIH | National Institute on Drug Abuse (NIDA)
- R01 DA025646/DA/NIDA NIH HHS/United States
- NS105602/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R01 MH116526/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources

