Expectations, needs and mid-term outcomes in people accessing to secondary findings from ES: 1st French mixed study (FIND Study)
- PMID: 38802530
- PMCID: PMC11368951
- DOI: 10.1038/s41431-024-01616-9
Expectations, needs and mid-term outcomes in people accessing to secondary findings from ES: 1st French mixed study (FIND Study)
Abstract
Generation and subsequently accessibility of secondary findings (SF) in diagnostic practice is a subject of debate around the world and particularly in Europe. The French FIND study has been set up to assess patient/parent expectations regarding SF from exome sequencing (ES) and to collect their real-life experience until 1 year after the delivery of results. 340 patients who had ES for undiagnosed developmental disorders were included in this multicenter mixed study (quantitative N = 340; qualitative N = 26). Three groups of actionable SF were rendered: predisposition to late-onset actionable diseases; genetic counseling; pharmacogenomics. Participants expressed strong interest in obtaining SF and a high satisfaction level when a SF is reported. The medical actionability of the SF reinforced parents' sense of taking action for their child and was seen as an opportunity. While we observed no serious psychological concerns, we showed that these results could have psychological consequences, in particular for late-onset actionable diseases SF, within families already dealing with rare diseases. This study shows that participants remain in favor of accessing SF despite the potential psychological, care, and lifestyle impacts, which are difficult to anticipate. The establishment of a management protocol, including the support of a multidisciplinary team, would be necessary if national policy allows the reporting of these data.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interest.
Figures

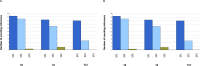
References
-
- Miller DT, Lee K, Gordon AS, Amendola LM, Adelman K, Bale SJ, et al. Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2021 update: a policy statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2021;23:1391–8. 10.1038/s41436-021-01171-4 - DOI - PMC - PubMed
-
- Miller DT, Lee K, Abul-Husn NS, Amendola LM, Brothers K, Chung WK, et al. ACMG SF v3.1 list for reporting of secondary findings in clinical exome and genome sequencing: a policy statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med J Am Coll Med Genet. 2022;24:1407–14. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

