Extracellular vesicles containing MFGE8 from colorectal cancer facilitate macrophage efferocytosis
- PMID: 38802814
- PMCID: PMC11131254
- DOI: 10.1186/s12964-024-01669-9
Extracellular vesicles containing MFGE8 from colorectal cancer facilitate macrophage efferocytosis
Abstract
Background: Colorectal cancer (CRC) commonly exhibits tolerance to cisplatin treatment, but the underlying mechanisms remain unclear. Within the tumor microenvironment, macrophages play a role in resisting the cytotoxic effects of chemotherapy by engaging in efferocytosis to clear apoptotic cells induced by chemotherapeutic agents. The involvement of extracellular vesicles (EVs), an intercellular communicator within the tumor microenvironment, in regulating the efferocytosis for the promotion of drug resistance has not been thoroughly investigated.
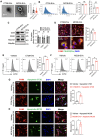
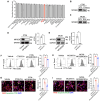
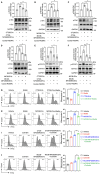
Methods: We constructed GFP fluorescent-expressing CRC cell lines (including GFP-CT26 and GFP-MC38) to detect macrophage efferocytosis through flow cytometric analysis. We isolated and purified CRC-secreted EVs using a multi-step ultracentrifugation method and identified them through electron microscopy and nanoflow cytometry. Proteomic analysis was conducted to identify the protein molecules carried by CRC-EVs. MFGE8 knockout CRC cell lines were constructed using CRISPR-Cas9, and their effects were validated through in vitro and in vivo experiments using Western blotting, immunofluorescence, and flow cytometric analysis, confirming that these EVs activate the macrophage αvβ3-Src-FAK-STAT3 signaling pathway, thereby promoting efferocytosis.
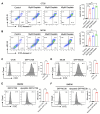
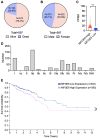
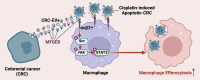
Results: In this study, we found that CRC-derived EVs (CRC-EVs) enhanced macrophage efferocytosis of cisplatin-induced apoptotic CRC cells. Analysis of The Cancer Genome Atlas (TCGA) database revealed a high expression of the efferocytosis-associated gene MFGE8 in CRC patients, suggesting a poorer prognosis. Additionally, mass spectrometry-based proteomic analysis identified a high abundance of MFGE8 protein in CRC-EVs. Utilizing CRISPR-Cas9 gene edition system, we generated MFGE8-knockout CRC cells, demonstrating that their EVs fail to upregulate macrophage efferocytosis in vitro and in vivo. Furthermore, we demonstrated that MFGE8 in CRC-EVs stimulated macrophage efferocytosis by increasing the expression of αvβ3 on the cell surface, thereby activating the intracellular Src-FAK-STAT3 signaling pathway.
Conclusions: Therefore, this study highlighted a mechanism in CRC-EVs carrying MFGE8 activated the macrophage efferocytosis. This activation promoted the clearance of cisplatin-induced apoptotic CRC cells, contributing to CRC resistance against cisplatin. These findings provide novel insights into the potential synergistic application of chemotherapy drugs, EVs inhibitors, and efferocytosis antagonists for CRC treatment.
Keywords: Colorectal cancer; Efferocytosis; Extracellular vesicle; MFGE8; Macrophage.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- JCYJ20230807110316034/Shenzhen Science and Technology Innovation Program
- 32200638/National Natural Science Foundation of China
- 2023A1515010090/Basic and Applied Basic Research Foundation of Guangdong Province
- ZSQYBRJH0021/Research Start-up Fund of the Seventh Affiliated Hospital, Sun Yat-sen University
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

