Long-Term Safety Analysis of a Fibrinogen Concentrate (RiaSTAP®/Haemocomplettan® P)
- PMID: 38803191
- PMCID: PMC11135097
- DOI: 10.1177/10760296241254106
Long-Term Safety Analysis of a Fibrinogen Concentrate (RiaSTAP®/Haemocomplettan® P)
Abstract
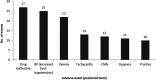
Fibrinogen concentrate treatment is recommended for acute bleeding episodes in adult and pediatric patients with congenital and acquired fibrinogen deficiency. Previous studies have reported a low risk of thromboembolic events (TEEs) with fibrinogen concentrate use; however, the post-treatment TEE risk remains a concern. A retrospective evaluation of RiaSTAP®/Haemocomplettan® P (CSL Behring, Marburg, Germany) post-marketing data was performed (January 1986-June 2022), complemented by a literature review of published studies. Approximately 7.45 million grams of fibrinogen concentrate was administered during the review period. Adverse drug reactions (ADRs) were reported in 337 patients, and 81 (24.0%) of these patients experienced possible TEEs, including 14/81 (17.3%) who experienced fatal outcomes. Risk factors and the administration of other coagulation products existed in most cases, providing alternative explanations. The literature review identified 52 high-ranking studies with fibrinogen concentrate across various clinical areas, including 26 randomized controlled trials. Overall, a higher number of comparative studies showed lower rates of ADRs and/or TEEs in the fibrinogen group versus the comparison group(s) compared with those that reported higher rates or no differences between groups. Post-marketing data and clinical studies demonstrate a low rate of ADRs, including TEEs, with fibrinogen concentrate treatment. These findings suggest a favorable safety profile of fibrinogen concentrate, placing it among the first-line treatments effective for managing intraoperative hemostatic bleeding.
Keywords: adverse drug reaction; fibrinogen concentrate; post-marketing; safety.
Figures
Similar articles
-
Safety of fibrinogen concentrate: analysis of more than 27 years of pharmacovigilance data.Thromb Haemost. 2015 Apr;113(4):759-71. doi: 10.1160/TH14-06-0514. Epub 2014 Dec 11. Thromb Haemost. 2015. PMID: 25502954 Review.
-
Fibrinogen Concentrate as an Alternative to Cryoprecipitate in a Postcardiopulmonary Transfusion Algorithm in Infants Undergoing Cardiac Surgery: A Prospective Randomized Controlled Trial.Anesth Analg. 2020 Mar;130(3):740-751. doi: 10.1213/ANE.0000000000004384. Anesth Analg. 2020. PMID: 31490252 Clinical Trial.
-
Pharmacokinetics, clot strength and safety of a new fibrinogen concentrate: randomized comparison with active control in congenital fibrinogen deficiency.J Thromb Haemost. 2018 Feb;16(2):253-261. doi: 10.1111/jth.13923. Epub 2018 Jan 22. J Thromb Haemost. 2018. PMID: 29220876 Clinical Trial.
-
Observational study of fibrinogen concentrate in massive hemorrhage: evaluation of a multicenter register.Blood Coagul Fibrinolysis. 2011 Dec;22(8):727-34. doi: 10.1097/MBC.0b013e32834cb343. Blood Coagul Fibrinolysis. 2011. PMID: 22024795
-
Treatment of congenital fibrinogen deficiency: overview and recent findings.Vasc Health Risk Manag. 2009;5:843-8. doi: 10.2147/vhrm.s5305. Epub 2009 Oct 12. Vasc Health Risk Manag. 2009. PMID: 19851522 Free PMC article. Review.
Cited by
-
Efficacy and safety of human fibrinogen concentrate (BT524) in patients with major haemorrhage undergoing major orthopaedic or abdominal surgery (AdFIrst): a randomised, active-controlled, multicentre, partially blinded, phase 3 non-inferiority trial.EClinicalMedicine. 2025 Jun 7;85:103264. doi: 10.1016/j.eclinm.2025.103264. eCollection 2025 Jul. EClinicalMedicine. 2025. PMID: 40741224 Free PMC article.
-
Administration of fibrinogen concentrates to patients with severe bleeding. How much is enough?Blood Transfus. 2025 Jan;23(1):75-78. doi: 10.2450/BloodTransfus.927. Blood Transfus. 2025. PMID: 39977524 Free PMC article. No abstract available.
References
-
- Kreuz W, Meili E, Peter-Salonen K, et al. Efficacy and tolerability of a pasteurised human fibrinogen concentrate in patients with congenital fibrinogen deficiency. Transfus Apher Sci. 2005;32(3):247‐253. - PubMed
-
- Blome M, Isgro F, Kiessling AH, et al. Relationship between factor XIII activity, fibrinogen, haemostasis screening tests and postoperative bleeding in cardiopulmonary bypass surgery. Thromb Haemost. 2005;93(6):1101‐1107. - PubMed
-
- Essa Y, Zeynalov N, Sandhaus T, Hofmann M, Lehmann T, Doenst T. Low fibrinogen is associated with increased bleeding-related re-exploration after cardiac surgery. Thorac Cardiovasc Surg. 2018;66(8):622‐628. - PubMed
-
- Cortet M, Deneux-Tharaux C, Dupont C, et al. Association between fibrinogen level and severity of postpartum haemorrhage: secondary analysis of a prospective trial. Br J Anaesth. 2012;108(6):984‐989. - PubMed
-
- Lier H, Krep H, Schroeder S, Stuber F. Preconditions of hemostasis in trauma: a review. The influence of acidosis, hypocalcemia, anemia, and hypothermia on functional hemostasis in trauma. J Trauma. 2008;65(4):951‐960. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources