Cytisine for smoking cessation: A 40-day treatment with an induction period
- PMID: 38803387
- PMCID: PMC11129281
- DOI: 10.18332/tpc/187556
Cytisine for smoking cessation: A 40-day treatment with an induction period
Abstract
Introduction: Cytisine is a smoking cessation drug now used worldwide. Most of the data available in the literature predict a 25-day treatment, accepted on the basis of previous clinical experience in Eastern Europe. There are few studies on dosing, and only recently some researchers have tried a longer treatment period.
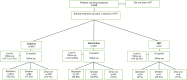
Methods: This real-world retrospective cross-sectional study analyzed data collected consecutively from 2015 to 2021, in seven smoking cessation centers in north-central Italy. The aim of this study is to evaluate the effectiveness and tolerability of a 40-day cytisine treatment with an induction phase and a slower reduction schedule. Data were collected from a group of 871 patients treated with cysteine, varenicline, and nicotine replacement therapy (NRT). The sample was not randomized. Behavioral support (4-6 sessions, each lasting 20-30 min, plus the evaluation session) was delivered to all patients.
Results: Subgroups taking cytisine (n=543 for 40 days), varenicline (n=281 for 12 weeks), and NRT (n=47 for eight weeks) showed biochemically confirmed smoking abstinence at 6 months of 50.5%, 55.9%, and 51.0%, respectively, with a statistically significant difference between cytisine versus varenicline (p<0.01) but not between cytisine versus NRT (p=0.5597). Adverse events were 4.4% with cytisine and 33.3% with varenicline. Behavioral support was an important factor in effectiveness.
Conclusions: This study produced preliminary evidence that the 40-day regimen of cytisine, appears to have less effectiveness in comparison to varenicline but the magnitude of the effect is comparable. The results and tolerability seem to be better than in most other studies.
Keywords: cytisine; nicotine; smoking cessation; tobacco smoke; varenicline.
© 2024 Tinghino B. et al.
Conflict of interest statement
The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none was reported.
Figures
References
-
- Pabreza LA, Dhawan S and Kellar KJ. Cytisine binding to nicotinic cholinergic receptors in the brain. Mol Pharmacol. 1991;39:9-12 - PubMed
-
- Papke RL, Heinemann S F. Partial agonist properties of cytisine on neuronal nicotinic receptors containing the beta 2 subunit. Mol Pharmacol 1994;45:142-149 - PubMed
LinkOut - more resources
Full Text Sources