Post-marketing risk analysis of bendamustine: a real-world approach based on the FAERS database
- PMID: 38803441
- PMCID: PMC11128657
- DOI: 10.3389/fphar.2024.1372401
Post-marketing risk analysis of bendamustine: a real-world approach based on the FAERS database
Abstract
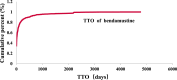
Objective: Bendamustine was approved for treating chronic lymphocytic leukemia and indolent B-cell non-Hodgkin lymphoma. Despite its therapeutic benefits, the long-term safety of bendamustine in a large population remains inadequately understood. This study evaluates the adverse events (AEs) associated with bendamustine, using a real-world pharmacovigilance database to support its clinical application. Methods: We conducted a post-marketing risk analysis to assess the association between bendamustine and its AEs. Data were extracted from the US FDA's Adverse Event Reporting System (FAERS), covering the period from January 2017 to September 2023. The characteristics of bendamustine-associated AEs and the onset time were further analyzed. Statistical analysis was performed using MYSQL 8.0, Navicat Premium 15, Microsoft EXCEL 2016, and Minitab 21.0. Results: 9,461,874 reports were collected from the FAERS database, 9,131 identified bendamustine as the "primary suspected" drug. We identified 331 significant disproportionality preferred terms (PTs). Common AEs included pyrexia, neutropenia, infusion site reaction, progressive multifocal leukoencephalopathy (PML), injection site vasculitis, and pneumonia-all documented on bendamustine's label. Notably, 16 unexpected and significant AEs were discovered, including hypogammaglobulinemia, which is concerning due to its potential to increase infection susceptibility following bendamustine treatment. Other significant findings were anaphylactic reactions, PML, and cutaneous malignancies, suggesting updates to the drug's label may be necessary. Physicians should monitor for neurological and skin changes in patients and discontinue treatment if PML is suspected. Moreover, the median onset time for bendamustine-associated AEs was 13 days, with an interquartile range [IQR] of 0-59 days, predominantly occurring on the first day post-initiation. The β of bendamustine-related AEs suggested risk reduction over time. Conclusion: Our study uncovered some potential pharmacovigilance signals for bendamustine, providing important insights for its safe and effective clinical use.
Keywords: FAERS; adverse events; bendamustine; data mining; pharmacovigilance; post-marketing risk analysis.
Copyright © 2024 Li, Zhang, Ni, Zhu, Lu, Chen, Cheng, Wang, Li, Wang, Lu, Chen and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Post-marketing safety of anti-IL-5 monoclonal antibodies (mAbs): an analysis of the FDA Adverse Event Reporting System (FAERS).Expert Opin Drug Saf. 2024 Mar;23(3):353-362. doi: 10.1080/14740338.2023.2251382. Epub 2023 Aug 29. Expert Opin Drug Saf. 2024. PMID: 37610085
-
A disproportionality analysis of adverse events associated to pertuzumab in the FDA Adverse Event Reporting System (FAERS).BMC Pharmacol Toxicol. 2023 Nov 13;24(1):62. doi: 10.1186/s40360-023-00702-w. BMC Pharmacol Toxicol. 2023. PMID: 37957717 Free PMC article.
-
A real-world pharmacovigilance study of FDA adverse event reporting system (FAERS) events for niraparib.Sci Rep. 2022 Nov 29;12(1):20601. doi: 10.1038/s41598-022-23726-4. Sci Rep. 2022. PMID: 36446798 Free PMC article.
-
A real-world pharmacovigilance study of FDA Adverse Event Reporting System (FAERS) events for osimertinib.Sci Rep. 2022 Nov 15;12(1):19555. doi: 10.1038/s41598-022-23834-1. Sci Rep. 2022. PMID: 36380085 Free PMC article.
-
Bendamustine for the treatment of chronic lymphocytic leukemia and rituximab-refractory, indolent B-cell non-Hodgkin lymphoma.Clin Ther. 2009;31 Pt 2:2290-311. doi: 10.1016/j.clinthera.2009.11.031. Clin Ther. 2009. PMID: 20110042 Review.
Cited by
-
Hypoglycemia associated with fluoroquinolone: a pharmacovigilance analysis from 2014 to 2023 based on the FDA adverse event reporting system.Front Med (Lausanne). 2025 May 2;12:1583093. doi: 10.3389/fmed.2025.1583093. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40385584 Free PMC article.
References
LinkOut - more resources
Full Text Sources

