Case report: Donor-derived CLL-1 chimeric antigen receptor T-cell therapy for relapsed/refractory acute myeloid leukemia bridging to allogeneic hematopoietic stem cell transplantation after remission
- PMID: 38803489
- PMCID: PMC11128603
- DOI: 10.3389/fimmu.2024.1389227
Case report: Donor-derived CLL-1 chimeric antigen receptor T-cell therapy for relapsed/refractory acute myeloid leukemia bridging to allogeneic hematopoietic stem cell transplantation after remission
Abstract
Background: Explore the efficacy and safety of donor-derived CLL-1 chimeric antigen receptor T-cell therapy (CAR-T) for relapsed/refractory acute myeloid leukemia (R/R AML) bridging to allogeneic hematopoietic stem cell transplantation (allo-HSCT) after remission.
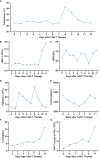
Case presentation: An adult R/R AML patient received an infusion of donor-derived CLL-1 CAR-T cells, and the conditioning regimen bridging to allo-HSCT was started immediately after remission on day 11 after CAR-T therapy upon transplantation. Then, routine post-HSCT monitoring of blood counts, bone marrow (BM) morphology, flow cytometry, graft-versus-host disease (GVHD) manifestations, and chimerism status were performed.
Result: After CAR-T therapy, cytokine release syndrome was grade 1. On day 11 after CAR-T therapy, the BM morphology reached complete remission (CR), and the conditioning regimen bridging to allo-HSCT started. Leukocyte engraftment, complete donor chimerism, and platelet engraftment were observed on days +18, +23, and +26 post-allo-HSCT, respectively. The BM morphology showed CR and flow cytometry turned negative on day +23. The patient is currently at 4 months post-allo-HSCT with BM morphology CR, negative flow cytometry, complete donor chimerism, and no extramedullary relapse/GVHD.
Conclusion: Donor-derived CLL-1 CAR-T is an effective and safe therapy for R/R AML, and immediate bridging to allo-HSCT after remission may better improve the long-term prognosis of R/R AML.
Keywords: C-type lectin-like molecule 1; acute myeloid leukemia; allogeneic hematopoietic stem cell transplantation; donor-derived chimeric antigen receptor T cells; relapsed/refractory.
Copyright © 2024 Miao, Shuai, Han, Zhang, Liu, Yao, Wang, He, Chen, Fan, Chang, Su and Yi.
Conflict of interest statement
Author AC is a founding member of Shanghai YaKe Biotechnology Ltd., a biotechnology company focusing on research and development of tumor cellular immunotherapy. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

