Disturbance of the human gut microbiota in patients with Myotonic Dystrophy type 1
- PMID: 38803516
- PMCID: PMC11128782
- DOI: 10.1016/j.csbj.2024.05.009
Disturbance of the human gut microbiota in patients with Myotonic Dystrophy type 1
Abstract
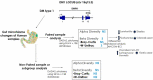
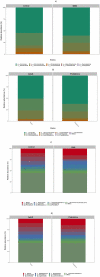
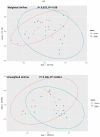
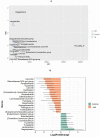
Myotonic dystrophy type 1 (DM1) is a rare autosomal dominant genetic disorder. Although DM1 is primarily characterized by progressive muscular weakness, it exhibits many multisystemic manifestations, such as cognitive deficits, cardiac conduction abnormalities, and cataracts, as well as endocrine and reproductive issues. Additionally, the gastrointestinal (GI) tract is frequently affected, encompassing the entire digestive tract. However, the underlying causes of these GI symptoms remain uncertain, whether it is biomechanical problems of the intestine, involvement of bacterial communities, or both. The primary objective of this study is to investigate the structural changes in the gut microbiome of DM1 patients. To achieve this purpose, 35 patients with DM1 were recruited from the DM-Scope registry of the neuromuscular clinic in the Saguenay-Lac-St-Jean region of the province of Québec, Canada. Stool samples from these 35 patients, including 15 paired samples with family members living with them as controls, were collected. Subsequently, these samples were sequenced by 16S MiSeq and were analyzed with DADA2 to generate taxonomic signatures. Our analysis revealed that the DM1 status correlated with changes in gut bacterial community. Notably, there were differences in the relative abundance of Bacteroidota, Euryarchaeota, Fusobacteriota, and Cyanobacteria Phyla compared to healthy controls. However, no significant shift in gut microbiome community structure was observed between DM1 phenotypes. These findings provide valuable insights into how the gut bacterial community, in conjunction with biomechanical factors, could potentially influence the gastrointestinal tract of DM1 patients.
Keywords: Bacterial communities; DM1; Gastrointestinal symptoms; Gut microbiota; Microbiome; Myotonic Dystrophy type 1.
© 2024 The Authors.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Influence of CTG repeats from the human DM1 locus on murine gut microbiota.Comput Struct Biotechnol J. 2025 Feb 19;27:733-743. doi: 10.1016/j.csbj.2025.02.016. eCollection 2025. Comput Struct Biotechnol J. 2025. PMID: 40092662 Free PMC article.
-
Consensus-Based Care Recommendations for Pulmonologists Treating Adults with Myotonic Dystrophy Type 1.Respiration. 2020;99(4):360-368. doi: 10.1159/000505634. Epub 2020 Apr 16. Respiration. 2020. PMID: 32299079 Review.
-
Patient engagement in clinical trial design for rare neuromuscular disorders: impact on the DELIVER and ACHIEVE clinical trials.Res Involv Engagem. 2024 Jan 2;10(1):1. doi: 10.1186/s40900-023-00535-1. Res Involv Engagem. 2024. PMID: 38167117 Free PMC article.
-
Utility and Results from a Patient-Reported Online Survey in Myotonic Dystrophies Types 1 and 2.Eur Neurol. 2020;83(5):523-533. doi: 10.1159/000511237. Epub 2020 Oct 29. Eur Neurol. 2020. PMID: 33120389
-
Clinical aspects, molecular pathomechanisms and management of myotonic dystrophies.Acta Myol. 2013 Dec;32(3):154-65. Acta Myol. 2013. PMID: 24803843 Free PMC article. Review.
Cited by
-
The Gut Microbiota Involvement in the Panorama of Muscular Dystrophy Pathogenesis.Int J Mol Sci. 2024 Oct 21;25(20):11310. doi: 10.3390/ijms252011310. Int J Mol Sci. 2024. PMID: 39457092 Free PMC article. Review.
-
Multisystem Symptoms in Myotonic Dystrophy Type 1: A Management and Therapeutic Perspective.Int J Mol Sci. 2025 Jun 2;26(11):5350. doi: 10.3390/ijms26115350. Int J Mol Sci. 2025. PMID: 40508159 Free PMC article. Review.
-
Influence of CTG repeats from the human DM1 locus on murine gut microbiota.Comput Struct Biotechnol J. 2025 Feb 19;27:733-743. doi: 10.1016/j.csbj.2025.02.016. eCollection 2025. Comput Struct Biotechnol J. 2025. PMID: 40092662 Free PMC article.
References
-
- Harper P.S. Myotonic Dystrophy. 3rd ed. London; Philadelphia, PA: W.B. Saunders; 2001.
-
- Meola G., Cardani R. Myotonic dystrophies: an update on clinical aspects, genetic, pathology, and molecular pathomechanisms. Biochim Et Biophys Acta (BBA) - Mol Basis Dis. 2015;1852(4):594–606. - PubMed
-
- Fisette-Paulhus I., Gagnon C., Girard-Côté L., Morin M. Genitourinary and lower gastrointestinal conditions in patients with myotonic dystrophy type 1: a systematic review of evidence and implications for clinical practice. Neuromuscul Disord. 2022;32(5):361–376. - PubMed
LinkOut - more resources
Full Text Sources
