Traditional Plant-Derived Compounds Inhibit Cell Migration and Induce Novel Cytoskeletal Effects in Glioblastoma Cells
- PMID: 38804289
- PMCID: PMC11130960
- DOI: 10.3390/jox14020036
Traditional Plant-Derived Compounds Inhibit Cell Migration and Induce Novel Cytoskeletal Effects in Glioblastoma Cells
Abstract
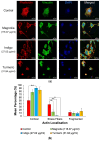
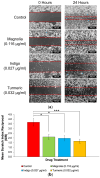
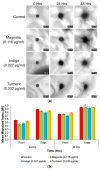
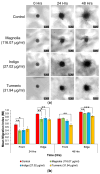
Glioblastomas (GBMs) are aggressive and invasive cancers of the brain, associated with high rates of tumour recurrence and poor patient outcomes despite initial treatment. Targeting cell migration is therefore of interest in highly invasive cancers such as GBMs, to prevent tumour dissemination and regrowth. One current aim of GBM research focuses on assessing the anti-migratory properties of novel or repurposed inhibitors, including plant-based drugs which display anti-cancer properties. We investigated the potential anti-migratory activity of plant-based products with known cytotoxic effects in cancers, using a range of two-dimensional (2D) and three-dimensional (3D) migration and invasion assays as well as immunofluorescence microscopy to determine the specific anti-migratory and phenotypic effects of three plant-derived compounds, Turmeric, Indigo and Magnolia bark, on established glioma cell lines. Migrastatic activity was observed in all three drugs, with Turmeric exerting the most inhibitory effect on GBM cell migration into scratches and from the spheroid edge at all the timepoints investigated (p < 0.001). We also observed novel cytoskeletal phenotypes affecting actin and the focal adhesion dynamics. As our in vitro results determined that Turmeric, Indigo and Magnolia are promising migrastatic drugs, we suggest additional experimentation at the whole organism level to further validate these novel findings.
Keywords: 2D/3D assays; anti-migratory; glioblastoma; indigo; invasion; magnolia; migration; turmeric.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
LinkOut - more resources
Full Text Sources
Research Materials

