Blood Vitamin C Levels of Patients Receiving Immunotherapy and Relationship to Monocyte Subtype and Epigenetic Modification
- PMID: 38804366
- PMCID: PMC11130941
- DOI: 10.3390/epigenomes8020017
Blood Vitamin C Levels of Patients Receiving Immunotherapy and Relationship to Monocyte Subtype and Epigenetic Modification
Abstract
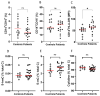
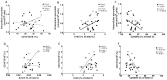
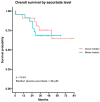
The treatment of metastatic melanoma has been revolutionised by immunotherapy, yet a significant number of patients do not respond, and many experience autoimmune adverse events. Associations have been reported between patient outcome and monocyte subsets, whereas vitamin C (ascorbate) has been shown to mediate changes in cancer-stimulated monocytes in vitro. We therefore investigated the relationship of ascorbate with monocyte subsets and epigenetic modifications in patients with metastatic melanoma receiving immunotherapy. Patients receiving immunotherapy were compared to other cancer cohorts and age-matched healthy controls. Ascorbate levels in plasma and peripheral blood-derived mononuclear cells (PBMCs), monocyte subtype and epigenetic markers were measured, and adverse events, tumour response and survival were recorded. A quarter of the immunotherapy cohort had hypovitaminosis C, with plasma and PBMC ascorbate levels significantly lower than those from other cancer patients or healthy controls. PBMCs from the immunotherapy cohort contained similar frequencies of non-classical and classical monocytes. DNA methylation markers and intracellular ascorbate concentration were correlated with monocyte subset frequency in healthy controls, but correlation was lost in immunotherapy patients. No associations between ascorbate status and immune-related adverse events or tumour response or overall survival were apparent.
Keywords: DNA methylation; ascorbate; metastatic melanoma; monocytes.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Cancer Society Melanoma Survival Rates|Melanoma Survival Statistics. [(accessed on 7 December 2023)]. Available online: https://www.cancer.org/cancer/melanoma-skin-cancer/detection-diagnosis-s....
-
- Huang J., Chan S.C., Ko S., Lok V., Zhang L., Lin X., Lucero-Prisno D.E., III, Xu W., Zheng Z.J., Elcarte E., et al. Global incidence, mortality, risk factors and trends of melanoma: A systematic analysis of registries. Am. J. Clin. Dermatol. 2023;24:965–975. doi: 10.1007/s40257-023-00795-3. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

