Prospects and Challenges in Developing mRNA Vaccines for Infectious Diseases and Oncogenic Viruses
- PMID: 38804384
- PMCID: PMC11130901
- DOI: 10.3390/medsci12020028
Prospects and Challenges in Developing mRNA Vaccines for Infectious Diseases and Oncogenic Viruses
Abstract
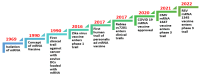
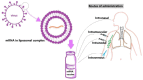
mRNA vaccines have emerged as an optimistic technological platform for vaccine innovation in this new scientific era. mRNA vaccines have dramatically altered the domain of vaccinology by offering a versatile and rapid approach to combating infectious diseases and virus-induced cancers. Clinical trials have demonstrated efficacy rates of 94-95% in preventing COVID-19, and mRNA vaccines have been increasingly recognized as a powerful vaccine platform. Although mRNA vaccines have played an essential role in the COVID-19 pandemic, they still have several limitations; their instability and degradation affect their storage, delivery, and over-all efficiency. mRNA is typically enclosed in a transport mechanism to facilitate its entry into the target cell because it is an unstable and negatively charged molecule. For instance, mRNA that is given using lipid-nanoparticle-based vaccine delivery systems (LNPs) solely enters cells through endocytosis, establishing an endosome without damaging the cell membrane. The COVID-19 pandemic has accelerated the development of mRNA vaccine platforms used to treat and prevent several infectious diseases. This technology has the potential to change the future course of the disease by providing a safe and effective way to combat infectious diseases and cancer. A single-stranded genetic sequence found in mRNA vaccines instructs host cells to produce proteins inside ribosomes to elicit immunological responses and prepare the immune system to fight infections or cancer cells. The potential applications of mRNA vaccine technology are vast and can lead to the development of a preferred vaccine pattern. As a result, a new generation of vaccinations has gradually gained popularity and access to the general population. To adapt the design of an antigen, and even combine sequences from different variations in response to new changes in the viral genome, mRNA vaccines may be used. Current mRNA vaccines provide adequate safety and protection, but the duration of that protection can only be determined if further clinical research is conducted.
Keywords: infectious diseases; mRNA technology; vaccine research; virus induced cancers.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
The Rapid Development and Early Success of Covid 19 Vaccines Have Raised Hopes for Accelerating the Cancer Treatment Mechanism.Arch Razi Inst. 2021 Mar;76(1):1-6. doi: 10.22092/ari.2021.353761.1612. Epub 2021 Mar 1. Arch Razi Inst. 2021. PMID: 33818952 Free PMC article.
-
The Potential of mRNA Vaccines to Fight Against Viruses.Viral Immunol. 2024 Oct;37(8):383-391. doi: 10.1089/vim.2024.0047. Viral Immunol. 2024. PMID: 39418074 Review.
-
The Nanoparticle-Enabled Success of COVID-19 mRNA Vaccines and the Promise of Microneedle Platforms for Pandemic Vaccine Response.DNA Cell Biol. 2022 Jan;41(1):25-29. doi: 10.1089/dna.2021.0538. Epub 2021 Dec 24. DNA Cell Biol. 2022. PMID: 34958232 Free PMC article. Review.
-
mRNA Vaccines: Design Principles, Mechanisms, and Manufacturing-Insights From COVID-19 as a Model for Combating Infectious Diseases.Biotechnol J. 2025 Feb;20(2):e202400596. doi: 10.1002/biot.202400596. Biotechnol J. 2025. PMID: 39989260 Review.
-
A Single Immunization with Nucleoside-Modified mRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-CoV-2 in Mice.Immunity. 2020 Oct 13;53(4):724-732.e7. doi: 10.1016/j.immuni.2020.07.019. Epub 2020 Jul 30. Immunity. 2020. PMID: 32783919 Free PMC article.
Cited by
-
mRNA vaccine platforms: linking infectious disease prevention and cancer immunotherapy.Front Bioeng Biotechnol. 2025 Mar 12;13:1547025. doi: 10.3389/fbioe.2025.1547025. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40144393 Free PMC article. Review.
-
mRNA Fragmentation Pattern Detected by SHAPE.Curr Issues Mol Biol. 2024 Sep 16;46(9):10249-10258. doi: 10.3390/cimb46090610. Curr Issues Mol Biol. 2024. PMID: 39329962 Free PMC article.
-
Comprehensive analysis of lncRNAs and mRNAs revealed potential participants in the process of avian reovirus infection.Front Microbiol. 2025 Feb 5;16:1539903. doi: 10.3389/fmicb.2025.1539903. eCollection 2025. Front Microbiol. 2025. PMID: 39973927 Free PMC article.
-
Comparing Moderna's mRNA-1083 and Pfizer's dual-target mRNA vaccines for influenza and COVID-19.NPJ Vaccines. 2025 May 24;10(1):105. doi: 10.1038/s41541-025-01145-6. NPJ Vaccines. 2025. PMID: 40413212 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

