RNA Polymerase Inhibitor Enisamium for Treatment of Moderate COVID-19 Patients: A Randomized, Placebo-Controlled, Multicenter, Double-Blind Phase 3 Clinical Trial
- PMID: 38804439
- PMCID: PMC11130936
- DOI: 10.3390/arm92030021
RNA Polymerase Inhibitor Enisamium for Treatment of Moderate COVID-19 Patients: A Randomized, Placebo-Controlled, Multicenter, Double-Blind Phase 3 Clinical Trial
Abstract
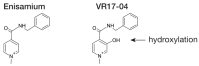
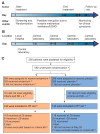
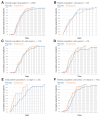
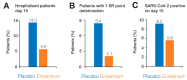
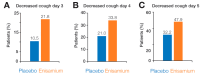
Enisamium is an orally available therapeutic that inhibits influenza A virus and SARS-CoV-2 replication. We evaluated the clinical efficacy of enisamium treatment combined with standard care in adult, hospitalized patients with moderate COVID-19 requiring external oxygen. Hospitalized patients with laboratory-confirmed SARS-CoV-2 infection were randomly assigned to receive either enisamium (500 mg per dose, four times a day) or a placebo. The primary outcome was an improvement of at least two points on an eight-point severity rating (SR) scale within 29 days of randomization. We initially set out to study the effect of enisamium on patients with a baseline SR of 4 or 5. However, because the study was started early in the COVID-19 pandemic, and COVID-19 had been insufficiently studied at the start of our study, an interim analysis was performed alongside a conditional power analysis in order to ensure patient safety and assess whether the treatment was likely to be beneficial for one or both groups. Following this analysis, a beneficial effect was observed for patients with an SR of 4 only, i.e., patients with moderate COVID-19 requiring supplementary oxygen. The study was continued for these COVID-19 patients. Overall, a total of 592 patients were enrolled and randomized between May 2020 and March 2021. Patients with a baseline SR of 4 were divided into two groups: 142 (49.8%) were assigned to the enisamium group and 143 (50.2%) to the placebo group. An analysis of the population showed that if patients were treated within 4 days of the onset of COVID-19 symptoms (n = 33), the median time to improvement was 8 days for the enisamium group and 13 days for the placebo group (p = 0.005). For patients treated within 10 days of the onset of COVID-19 symptoms (n = 154), the median time to improvement was 10 days for the enisamium group and 12 days for the placebo group (p = 0.002). Our findings suggest that enisamium is safe to use with COVID-19 patients, and that the observed clinical benefit of enisamium is worth reporting and studying in detail.
Keywords: Amizon; COVID-19; FAV00A; RNA polymerase; SARS-CoV-2; antiviral; clinical trial.
Conflict of interest statement
A.T.V. received grants from the National Institutes of Health, the Wellcome Trust, and the Royal Society. V.M. and A.G. are employees of Farmak JSC. J.M. and H.S. received personal fees from Pharmalog Institut für klinische Forschung GmbH as subcontracted service CRO. L.M. received personal fees from Regenold GmbH as a subcontracted consultant. A.J.W.t.V. was previously employed by the University of Cambridge. The University of Cambridge received consulting fees for the experiments and analyses performed by A.J.W.t.V.
Figures
References
-
- Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., et al. The Species Severe Acute Respiratory Syndrome-Related Coronavirus: Classifying 2019-nCoV and Naming It SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

