Inhibition of HDAC2 sensitises antitumour therapy by promoting NLRP3/GSDMD-mediated pyroptosis in colorectal cancer
- PMID: 38804602
- PMCID: PMC11131357
- DOI: 10.1002/ctm2.1692
Inhibition of HDAC2 sensitises antitumour therapy by promoting NLRP3/GSDMD-mediated pyroptosis in colorectal cancer
Abstract
Background: Although numerous studies have indicated that activated pyroptosis can enhance the efficacy of antitumour therapy in several tumours, the precise mechanism of pyroptosis in colorectal cancer (CRC) remains unclear.
Methods: Pyroptosis in CRC cells treated with antitumour agents was assessed using various techniques, including Western blotting, lactate dehydrogenase release assay and microscopy analysis. To uncover the epigenetic mechanisms that regulate NLRP3, chromatin changes and NLRP3 promoter histone modifications were assessed using Assay for Transposase-Accessible Chromatin using sequencing and RNA sequencing. Chromatin immunoprecipitation‒quantitative polymerase chain reaction was used to investigate the NLRP3 transcriptional regulatory mechanism. Additionally, xenograft and patient-derived xenograft models were constructed to validate the effects of the drug combinations.
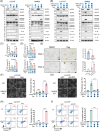
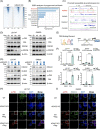
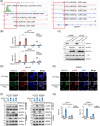
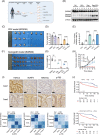
Results: As the core molecule of the inflammasome, NLRP3 expression was silenced in CRC, thereby limiting gasdermin D (GSDMD)-mediated pyroptosis. Supplementation with NLRP3 can rescue pyroptosis induced by antitumour therapy. Overexpression of HDAC2 in CRC silences NLRP3 via epigenetic regulation. Mechanistically, HDAC2 suppressed chromatin accessibility by eliminating H3K27 acetylation. HDAC2 knockout promotes H3K27ac-mediated recruitment of the BRD4-p-P65 complex to enhance NLRP3 transcription. Inhibiting HDAC2 by Santacruzamate A in combination with classic antitumour agents (5-fluorouracil or regorafenib) in CRC xenograft-bearing animals markedly activated pyroptosis and achieved a significant therapeutic effect. Clinically, HDAC2 is inversely correlated with H3K27ac/p-P65/NLRP3 and is a prognostic factor for CRC patients.
Conclusion: Collectively, our data revealed a crucial role for HDAC2 in inhibiting NLRP3/GSDMD-mediated pyroptosis in CRC cells and highlighted HDAC2 as a potential therapeutic target for antitumour therapy.
Highlights: Silencing of NLRP3 limits the GSDMD-dependent pyroptosis in colorectal cancer. HDAC2-mediated histone deacetylation leads to epigenetic silencing of NLRP3. HDAC2 suppresses the NLRP3 transcription by inhibiting the formation of H3K27ac/BRD4/p-P65 complex. Targeting HDAC2 activates pyroptosis and enhances therapeutic effect.
Keywords: H3K27ac; HDAC2; NLRP3; colorectal cancer; pyroptosis.
© 2024 The Author(s). Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare they have no conflicts of interest.
Figures



References
-
- Morgan E, Arnold M, Gini A, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. 2023;72:338‐344. - PubMed
-
- Ciardiello F, Ciardiello D, Martini G, Napolitano S, Tabernero J, Cervantes A. Clinical management of metastatic colorectal cancer in the era of precision medicine. CA Cancer J Clin. 2022;72:372‐401. - PubMed
-
- Cervantes A, Adam R, Roselló S, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow‐up. Ann Oncol. 2023;34:10‐32. - PubMed
-
- Grothey A, Blay JY, Pavlakis N, Yoshino T, Bruix J. Evolving role of regorafenib for the treatment of advanced cancers. Cancer Treat Rev. 2020;86:101993. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
