ATP1A3 regulates protein synthesis for mitochondrial stability under heat stress
- PMID: 38804677
- PMCID: PMC11247502
- DOI: 10.1242/dmm.050574
ATP1A3 regulates protein synthesis for mitochondrial stability under heat stress
Abstract
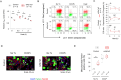
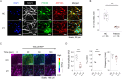
Pathogenic variants in ATP1A3, the gene encoding the α3 subunit of the Na+/K+-ATPase, cause alternating hemiplegia of childhood (AHC) and related disorders. Impairments in Na+/K+-ATPase activity are associated with the clinical phenotype. However, it remains unclear whether additional mechanisms are involved in the exaggerated symptoms under stressed conditions in patients with AHC. We herein report that the intracellular loop (ICL) of ATP1A3 interacted with RNA-binding proteins, such as Eif4g (encoded by Eif4g1), Pabpc1 and Fmrp (encoded by Fmr1), in mouse Neuro2a cells. Both the siRNA-mediated depletion of Atp1a3 and ectopic expression of the p.R756C variant of human ATP1A3-ICL in Neuro2a cells resulted in excessive phosphorylation of ribosomal protein S6 (encoded by Rps6) and increased susceptibility to heat stress. In agreement with these findings, induced pluripotent stem cells (iPSCs) from a patient with the p.R756C variant were more vulnerable to heat stress than control iPSCs. Neurons established from the patient-derived iPSCs showed lower calcium influxes in responses to stimulation with ATP than those in control iPSCs. These data indicate that inefficient protein synthesis contributes to the progressive and deteriorating phenotypes in patients with the p.R756C variant among a variety of ATP1A3-related disorders.
Keywords: Alternating hemiplegia of childhood; Heat stress; Induced pluripotent stem cells (iPSCs); Interaction; Mitochondria; Na+/K+-ATPase α3 subunit (ATP1A3); Protein synthesis.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures






Similar articles
-
Neuronal modeling of alternating hemiplegia of childhood reveals transcriptional compensation and replicates a trigger-induced phenotype.Neurobiol Dis. 2020 Jul;141:104881. doi: 10.1016/j.nbd.2020.104881. Epub 2020 Apr 27. Neurobiol Dis. 2020. PMID: 32348881
-
Direct evidence of impaired neuronal Na/K-ATPase pump function in alternating hemiplegia of childhood.Neurobiol Dis. 2018 Jul;115:29-38. doi: 10.1016/j.nbd.2018.03.009. Epub 2018 Mar 19. Neurobiol Dis. 2018. PMID: 29567111
-
De novo p.Arg756Cys mutation of ATP1A3 causes an atypical form of alternating hemiplegia of childhood with prolonged paralysis and choreoathetosis.BMC Neurol. 2016 Sep 15;16:174. doi: 10.1186/s12883-016-0680-6. BMC Neurol. 2016. PMID: 27634470 Free PMC article.
-
Insights into the Pathology of the α3 Na(+)/K(+)-ATPase Ion Pump in Neurological Disorders; Lessons from Animal Models.Front Physiol. 2016 Jun 14;7:209. doi: 10.3389/fphys.2016.00209. eCollection 2016. Front Physiol. 2016. PMID: 27378932 Free PMC article. Review.
-
Alternating Hemiplegia of Childhood: Understanding the Genotype-Phenotype Relationship of ATP1A3 Variations.Appl Clin Genet. 2020 Mar 30;13:71-81. doi: 10.2147/TACG.S210325. eCollection 2020. Appl Clin Genet. 2020. PMID: 32280259 Free PMC article. Review.
Cited by
-
Extracellular Vesicles for Clinical Diagnostics: From Bulk Measurements to Single-Vesicle Analysis.ACS Nano. 2025 Aug 12;19(31):28021-28109. doi: 10.1021/acsnano.5c00706. Epub 2025 Jul 28. ACS Nano. 2025. PMID: 40720603 Free PMC article. Review.
-
Targeting stress induction of GRP78 by cardiac glycoside oleandrin dually suppresses cancer and COVID-19.Cell Biosci. 2024 Sep 6;14(1):115. doi: 10.1186/s13578-024-01297-3. Cell Biosci. 2024. PMID: 39238058 Free PMC article.
-
Translating multiscale research in rare disease.Dis Model Mech. 2024 Jun 1;17(6):dmm052009. doi: 10.1242/dmm.052009. Epub 2024 Jul 10. Dis Model Mech. 2024. PMID: 38982973 Free PMC article.
References
-
- Arystarkhova, E., Haq, I. U., Luebbert, T., Mochel, F., Saunders-Pullman, R., Bressman, S. B., Feschenko, P., Salazar, C., Cook, J. F., Demarest, S.et al. (2019). Factors in the disease severity of ATP1A3 mutations: Impairment, misfolding, and allele competition. Neurobiol. Dis. 132, 104577. 10.1016/j.nbd.2019.104577 - DOI - PMC - PubMed
-
- Arystarkhova, E., Toustrup-Jensen, M. S., Holm, R., Ko, J. K., Lee, K. E., Feschenko, P., Ozelius, L. J., Brashear, A., Vilsen, B. and Sweadner, K. J. (2023). Temperature instability of a mutation at a multidomain junction in Na,K-ATPase isoform ATP1A3 (p.Arg756His) produces a fever-induced neurological syndrome. J. Biol. Chem. 299, 102758. 10.1016/j.jbc.2022.102758 - DOI - PMC - PubMed
-
- Barwe, S. P., Anilkumar, G., Moon, S. Y., Zheng, Y., Whitelegge, J. P., Rajasekaran, S. A. and Rajasekaran, A. K. (2005). Novel role for Na,K-ATPase in phosphatidylinositol 3-kinase signaling and suppression of cell motility. Mol. Biol. Cell 16, 1082-1094. 10.1091/mbc.e04-05-0427 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

