Exploratory study to evaluate the acceptability of a wearable accelerometer to assess motor progression in motor neuron disease
- PMID: 38805054
- PMCID: PMC11319372
- DOI: 10.1007/s00415-024-12449-3
Exploratory study to evaluate the acceptability of a wearable accelerometer to assess motor progression in motor neuron disease
Abstract
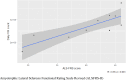
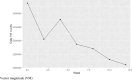
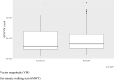
Motor neuron disease (MND) is a rapidly progressive condition traditionally assessed using a questionnaire to evaluate physical function, the revised amyotrophic lateral sclerosis functional rating scale (ALSFRS-R). Its use can be associated with poor sensitivity in detecting subtle changes over time and there is an urgent need for more sensitive and specific outcome measures. The ActiGraph GT9X is a wearable device containing multiple sensors that can be used to provide metrics that represent physical activity. The primary aim of this study was to investigate the initial suitability and acceptability of limb-worn wearable devices to group of people with MND in Scotland. A secondary aim was to explore the preliminary associations between the accelerometer sensor data within the ActiGraph GT9X and established measures of physical function. 10 participants with MND completed a 12-week schedule of assessments including fortnightly study visits, both in-person and over videoconferencing software. Participants wore the device on their right wrist and right ankle for a series of movements, during a 6-min walking test and for a period of 24-h wear, including overnight. Participants also completed an ALSFRS-R and questionnaires on their experience with the devices. 80% of the participants found wearing these devices to be a positive experience and no one reported interference with daily living or added burden. However, 30% of the participants experienced technical issues with their devices. Data from the wearable devices correlated with established measures of physical function.
Keywords: Accelerometer; Digital health; Motor neuron disease; Wearable devices.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
MeSH terms
LinkOut - more resources
Full Text Sources

