Transport activity regulates mitochondrial bioenergetics and biogenesis in renal tubules
- PMID: 38805156
- PMCID: PMC11147170
- DOI: 10.1096/fj.202400358RR
Transport activity regulates mitochondrial bioenergetics and biogenesis in renal tubules
Abstract
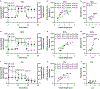
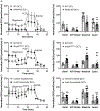
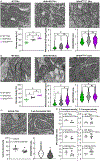
Renal tubules are featured with copious mitochondria and robust transport activity. Mutations in mitochondrial genes cause congenital renal tubulopathies, and changes in transport activity affect mitochondrial morphology, suggesting mitochondrial function and transport activity are tightly coupled. Current methods of using bulk kidney tissues or cultured cells to study mitochondrial bioenergetics are limited. Here, we optimized an extracellular flux analysis (EFA) to study mitochondrial respiration and energy metabolism using microdissected mouse renal tubule segments. EFA detects mitochondrial respiration and glycolysis by measuring oxygen consumption and extracellular acidification rates, respectively. We show that both measurements positively correlate with sample sizes of a few centimeter-length renal tubules. The thick ascending limbs (TALs) and distal convoluted tubules (DCTs) critically utilize glucose/pyruvate as energy substrates, whereas proximal tubules (PTs) are significantly much less so. Acute inhibition of TALs' transport activity by ouabain treatment reduces basal and ATP-linked mitochondrial respiration. Chronic inhibition of transport activity by 2-week furosemide treatment or deletion of with-no-lysine kinase 4 (Wnk4) decreases maximal mitochondrial capacity. In addition, chronic inhibition downregulates mitochondrial DNA mass and mitochondrial length/density in TALs and DCTs. Conversely, gain-of-function Wnk4 mutation increases maximal mitochondrial capacity and mitochondrial length/density without increasing mitochondrial DNA mass. In conclusion, EFA is a sensitive and reliable method to investigate mitochondrial functions in isolated renal tubules. Transport activity tightly regulates mitochondrial bioenergetics and biogenesis to meet the energy demand in renal tubules. The system allows future investigation into whether and how mitochondria contribute to tubular remodeling adapted to changes in transport activity.
Keywords: distal convoluted tubule; extracellular flux analysis; glycolysis; mitochondrial respiration; thick ascending limb; transport activity.
© 2024 Federation of American Societies for Experimental Biology.
Conflict of interest statement
Conflict of Interest Statement
The authors declare no conflicts of interest.
Figures







Update of
-
Transport activity regulates mitochondrial bioenergetics and biogenesis in renal tubules.bioRxiv [Preprint]. 2024 Feb 8:2024.02.04.578838. doi: 10.1101/2024.02.04.578838. bioRxiv. 2024. Update in: FASEB J. 2024 May 31;38(10):e23703. doi: 10.1096/fj.202400358RR. PMID: 38370657 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
- DK111542/HHS | NIH | NIDDK | Division of Diabetes, Endocrinology, and Metabolic Diseases (DEM)
- DK134420/HHS | NIH | NIDDK | Division of Diabetes, Endocrinology, and Metabolic Diseases (DEM)
- R01 DK111542/DK/NIDDK NIH HHS/United States
- R01 DK134420/DK/NIDDK NIH HHS/United States
- P50 HD103556/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

