Mobile Mindfulness Intervention for Psychological Distress Among Intensive Care Unit Survivors: A Randomized Clinical Trial
- PMID: 38805199
- PMCID: PMC11134280
- DOI: 10.1001/jamainternmed.2024.0823
Mobile Mindfulness Intervention for Psychological Distress Among Intensive Care Unit Survivors: A Randomized Clinical Trial
Abstract
Importance: Although psychological distress is common among survivors of critical illness, there are few tailored therapies.
Objective: To determine the optimal method for delivering a mindfulness intervention via a mobile app for critical illness survivors.
Design, setting, and participants: This randomized clinical trial used a 2 × 2 × 2 factorial design and was conducted at 3 sites among survivors of critical illness with elevated postdischarge symptoms of depression. The study was conducted between August 2019 and July 2023.
Interventions: Participants were randomized to 1 of 8 different groups as determined by 3 two-level intervention component combinations: intervention introduction method (mobile app vs therapist call), mindfulness meditation dose (once daily vs twice daily), and management of increasing symptoms (mobile app vs therapist call).
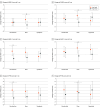
Main outcomes and measures: The primary outcome was the 9-item Patient Health Questionnaire (PHQ-9) depression scale score (range, 0-27) at 1 month. Secondary outcomes included anxiety (7-item Generalized Anxiety Disorder) and posttraumatic stress disorder (Posttraumatic Stress Scale) symptoms at 1 and 3 months, adherence, and feasibility. General linear models were used to compare main effects and interactions of the components among intervention groups. A formal decisional framework was used to determine an optimized intervention version.
Results: A total of 247 participants (mean [SD] age, 50.2 [15.4] years; 104 [42.1%] women) were randomized. Twice-daily meditation compared with once-daily meditation was associated with a 1.2 (95% CI, 0.04-2.4)-unit lower mean estimated PHQ-9 score at 1 month and a 1.5 (95% CI, 0.1-2.8)-unit lower estimated mean score at 3 months. The other 2 intervention components had no main effects on the PHQ-9. Across-group adherence was high (217 participants [87.9%] using the intervention at trial conclusion) and retention was strong (191 [77.3%] and 182 [73.7%] at 1 and 3 months, respectively).
Conclusions and relevance: A mindfulness intervention for survivors of critical illness that included an app-based introduction, twice-daily guided meditation, and app-based management of increasing depression symptoms was optimal considering effects on psychological distress symptoms, adherence, and feasibility.
Trial registration: ClinicalTrials.gov Identifier: NCT04038567.
Conflict of interest statement
Figures
Comment on
-
Value in Optimizing the Components of App Interventions Before Final Testing.JAMA Intern Med. 2024 Jul 1;184(7):760. doi: 10.1001/jamainternmed.2024.0833. JAMA Intern Med. 2024. PMID: 38805201 No abstract available.
References
-
- Kamdar BB, Huang M, Dinglas VD, et al. ; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network . Joblessness and lost earnings after acute respiratory distress syndrome in a 1-year national multicenter study. Am J Respir Crit Care Med. 2017;196(8):1012-1020. doi:10.1164/rccm.201611-2327OC - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

