Early vs Late Anticoagulation in Minor, Moderate, and Major Ischemic Stroke With Atrial Fibrillation: Post Hoc Analysis of the ELAN Randomized Clinical Trial
- PMID: 38805207
- PMCID: PMC11134281
- DOI: 10.1001/jamaneurol.2024.1450
Early vs Late Anticoagulation in Minor, Moderate, and Major Ischemic Stroke With Atrial Fibrillation: Post Hoc Analysis of the ELAN Randomized Clinical Trial
Abstract
Importance: Whether infarct size modifies the treatment effect of early vs late direct oral anticoagulant (DOAC) initiation in people with ischemic stroke and atrial fibrillation is unknown.
Objective: To assess whether infarct size modifies the safety and efficacy of early vs late DOAC initiation.
Design, setting, and participants: Post hoc analysis of participants from the multinational (>100 sites in 15 countries) randomized clinical Early Versus Later Anticoagulation for Stroke With Atrial Fibrillation (ELAN) trial who had (1) acute ischemic stroke, (2) atrial fibrillation, and (3) brain imaging available before randomization. The ELAN trial was conducted between October 2017 and December 2022. Data were analyzed from October to December 2023 for this post hoc analysis.
Intervention: Early vs late DOAC initiation after ischemic stroke. Early DOAC initiation was within 48 hours for minor or moderate stroke or on days 6 to 7 for major stroke; late DOAC initiation was on days 3 to 4 for minor stroke, days 6 to 7 for moderate stroke, and days 12 to 14 for major stroke.
Main outcomes and measures: The primary outcome was a composite of recurrent ischemic stroke, symptomatic intracranial hemorrhage, extracranial bleeding, systemic embolism, or vascular death within 30 days. The outcome was assessed according to infarct size (minor, moderate, or major) using odds ratios and risk differences between treatment arms. Interrater reliability for infarct size between the core laboratory and local raters was assessed, and whether this modified the estimated treatment effects was also examined.
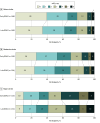
Results: A total of 1962 of the original 2013 participants (909 [46.3%] female; median [IQR] age, 77 [70-84] years) were included. The primary outcome occurred in 10 of 371 participants (2.7%) with early DOAC initiation vs 11 of 364 (3.0%) with late DOAC initiation among those with minor stroke (odds ratio [OR], 0.89; 95% CI, 0.38-2.10); in 11 of 388 (2.8%) with early DOAC initiation vs 14 of 392 (3.6%) with late DOAC initiation among those with moderate stroke (OR, 0.80; 95% CI, 0.35-1.74); and in 8 of 219 (3.7%) with early DOAC initiation vs 16 of 228 (7.0%) with late DOAC initiation among those with major stroke (OR, 0.52; 95% CI, 0.21-1.18). The 95% CI for the estimated risk difference of the primary outcome in early anticoagulation was -2.78% to 2.12% for minor stroke, -3.23% to 1.76% for moderate stroke, and -7.49% to 0.81% for major stroke. There was no significant treatment interaction for the primary outcome. For infarct size, interrater reliability was moderate (κ = 0.675; 95% CI, 0.647-0.702) for local vs core laboratory raters and strong (κ = 0.875; 95% CI, 0.855-0.894) between core laboratory raters.
Conclusions and relevance: The treatment effect of early DOAC initiation did not differ in people with minor, moderate, or major stroke assessed by brain imaging. Early treatment was not associated with a higher rate of adverse events, especially symptomatic intracranial hemorrhage, for any infarct size, including major stroke.
Trial registration: ClinicalTrials.gov Identifier: NCT03148457.
Conflict of interest statement
Figures

References
-
- Seiffge DJ, Paciaroni M, Wilson D, et al. ; CROMIS-2, RAF, RAF-DOAC, SAMURAI, NOACISP LONGTERM, Erlangen, and Verona Registry Collaborators . Direct oral anticoagulants versus vitamin K antagonists after recent ischemic stroke in patients with atrial fibrillation. Ann Neurol. 2019;85(6):823-834. doi:10.1002/ana.25489 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

