Discovery of GLPG3667, a Selective ATP Competitive Tyrosine Kinase 2 Inhibitor for the Treatment of Autoimmune Diseases
- PMID: 38805213
- PMCID: PMC11181332
- DOI: 10.1021/acs.jmedchem.4c00769
Discovery of GLPG3667, a Selective ATP Competitive Tyrosine Kinase 2 Inhibitor for the Treatment of Autoimmune Diseases
Abstract
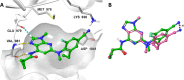
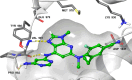
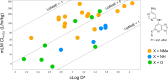
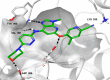
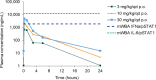
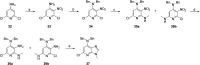
Tyrosine kinase 2 (TYK2) mediates cytokine signaling through type 1 interferon, interleukin (IL)-12/IL-23, and the IL-10 family. There appears to be an association between TYK2 genetic variants and inflammatory conditions, and clinical evidence suggests that selective inhibition of TYK2 could produce a unique therapeutic profile. Here, we describe the discovery of compound 9 (GLPG3667), a reversible and selective TYK2 adenosine triphosphate competitive inhibitor in development for the treatment of inflammatory and autoimmune diseases. The preclinical pharmacokinetic profile was favorable, and TYK2 selectivity was confirmed in peripheral blood mononuclear cells and whole blood assays. Dermal ear inflammation was reduced in an IL-23-induced in vivo mouse model of psoriasis. GLPG3667 also completed a phase 1b study (NCT04594928) in patients with moderate-to-severe psoriasis where clinical effect was shown within the 4 weeks of treatment and it is now in phase 2 trials for the treatment of dermatomyositis (NCT05695950) and systemic lupus erythematosus (NCT05856448).
Conflict of interest statement
The authors declare the following competing financial interest(s): All authors were employees of Galapagos NV at the time of the study. OM and JMJ are employees and shareholders of Janssen Pharmaceuticals. PC is an employee of Confo Therapeutics. RoB, CJ and FM are employees of NovAliX. SM, GC, SDV, LO, DA, MLR and RG are employees and shareholders of Galapagos NV. KS is an employee of Oncodesign Precision Medicine. MB was an employee of Galapagos at the time of the work. EQ is an employee and shareholder of AGC Pharma Chemicals Europe. ReB is an employee and shareholder of Agomab. SvdP is an employee and shareholder of iTeos Therapeutics.
Figures
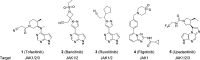
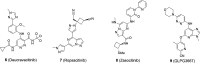


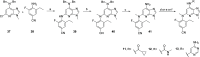
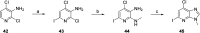

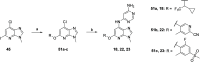

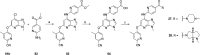
Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2020 Jan 9;1(1):CD011535. doi: 10.1002/14651858.CD011535.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 19;4:CD011535. doi: 10.1002/14651858.CD011535.pub4. PMID: 31917873 Free PMC article. Updated.
-
Pharmacological inhibition of tyrosine protein-kinase 2 reduces islet inflammation and delays type 1 diabetes onset in mice.EBioMedicine. 2025 Jul;117:105734. doi: 10.1016/j.ebiom.2025.105734. Epub 2025 May 6. EBioMedicine. 2025. PMID: 40335415 Free PMC article.
-
Oral small-molecule tyrosine kinase 2 and phosphodiesterase 4 inhibitors in plaque psoriasis: a network meta-analysis.Front Immunol. 2023 Jun 2;14:1180170. doi: 10.3389/fimmu.2023.1180170. eCollection 2023. Front Immunol. 2023. PMID: 37334353 Free PMC article.
Cited by
-
Advances in the treatment of systemic lupus erythematosus.Nat Rev Drug Discov. 2025 Jul 17. doi: 10.1038/s41573-025-01242-0. Online ahead of print. Nat Rev Drug Discov. 2025. PMID: 40676245 Review.
References
-
- Firmbach-Kraft I.; Byers M.; Shows T.; Dalla-Favera R.; Krolewski J. J. tyk2, prototype of a novel class of non-receptor tyrosine kinase genes. Oncogene 1990, 5 (9), 1329–1336. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

