Deletion of Hsd11b1 suppresses caloric restriction-induced bone marrow adiposity in male but not female mice
- PMID: 38805506
- PMCID: PMC11301425
- DOI: 10.1530/JOE-24-0072
Deletion of Hsd11b1 suppresses caloric restriction-induced bone marrow adiposity in male but not female mice
Abstract
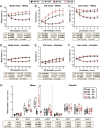
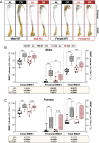
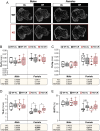
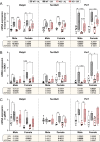
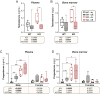
Bone marrow adipose tissue (BMAT) comprises >10% of total adipose mass in healthy humans. It increases in diverse conditions, including ageing, obesity, osteoporosis, glucocorticoid therapy, and notably, during caloric restriction (CR). BMAT potentially influences skeletal, metabolic, and immune functions, but the mechanisms of BMAT expansion remain poorly understood. Our hypothesis is that, during CR, excessive glucocorticoid activity drives BMAT expansion. The enzyme 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) amplifies glucocorticoid activity by catalysing intracellular regeneration of active glucocorticoids from inert 11-keto forms. Mice lacking 11β-HSD1 resist metabolic dysregulation and bone loss during exogenous glucocorticoid excess; thus, we hypothesised that 11β-HSD1 knockout mice would also resist excessive glucocorticoid action during CR, thereby restrining BMAT expansion and bone loss. To test this, we first confirmed that 11β-HSD1 is expressed in mouse and human bone marrow. We then investigated the effects of CR in male and female control and 11β-HSD1 knockout mice from 9 to 15 weeks of age. CR increased Hsd11b1 mRNA in adipose tissue and bone marrow. Deletion of Hsd11b1 did not alter bone or BMAT characteristics in mice fed a control diet and had little effect on tibial bone microarchitecture during CR. Notably, Hsd11b1 deletion attenuated the CR-induced increases in BMAT and prevented increases in bone marrow corticosterone in males but not females. This was not associated with suppression of glucocorticoid target genes in bone marrow. Instead, knockout males had increased progesterone in plasma and bone marrow. Together, our findings show that knockout of 11β-HSD1 prevents CR-induced BMAT expansion in a sex-specific manner and highlights progesterone as a potential new regulator of bone marrow adiposity.
Keywords: 11β-HSD1; bone; bone marrow adipose tissue; caloric restriction; glucocorticoids; progesterone; sex differences.
Conflict of interest statement
The authors declare that there are no conflicts of interest that could be perceived as prejudicing the impartiality of the research reported herein. KEC is a Senior Editor of the
Figures



References
-
- Bravenboer N, Bredella MA, Chauveau C, Corsi A, Douni E, Ferris WF, Riminucci M, Robey PG, Rojas-Sutterlin S, Rosen C, et al.2020Standardised nomenclature, abbreviations, and units for the study of bone marrow adiposity: report of the nomenclature working group of the international bone marrow adiposity society. Frontiers in Endocrinology 10. (10.3389/fendo.2019.00923) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

