Packaging of supplemented urokinase into alpha granules of in vitro-grown megakaryocytes for targeted nascent clot lysis
- PMID: 38805575
- PMCID: PMC11298819
- DOI: 10.1182/bloodadvances.2024012835
Packaging of supplemented urokinase into alpha granules of in vitro-grown megakaryocytes for targeted nascent clot lysis
Abstract
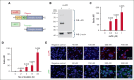
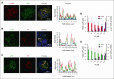
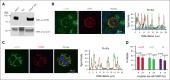
Fibrinolytics delivered into the general circulation lack selectivity for nascent thrombi, reducing efficacy and increasing the risk of bleeding. Urokinase-type plasminogen activator (uPA) transgenically expressed within murine platelets provided targeted thromboprophylaxis without causing bleeding but is not clinically feasible. Recent advances in generating megakaryocytes prompted us to develop a potentially clinically relevant means to produce "antithrombotic" platelets from CD34+ hematopoietic stem cell-derived in vitro-grown megakaryocytes. CD34+ megakaryocytes internalize and store in alpha granules (α-granules) single-chain uPA (scuPA) and a plasmin-resistant thrombin-activatable variant (uPAT). Both uPAs colocalized with internalized factor V (FV), fibrinogen and plasminogen, low-density lipoprotein receptor-related protein 1 (LRP1), and interferon-induced transmembrane protein 3, but not with endogenous von Willebrand factor (VWF). Endocytosis of uPA by CD34+ megakaryocytes was mediated, in part, via LRP1 and αIIbβ3. scuPA-containing megakaryocytes degraded endocytosed intragranular FV but not endogenous VWF in the presence of internalized plasminogen, whereas uPAT-megakaryocytes did not significantly degrade either protein. We used a carotid artery injury model in nonobese diabetic-severe combined immunodeficiency IL2rγnull (NSG) mice homozygous for VWFR1326H (a mutation switching binding VWF specificity from mouse to human glycoprotein Ibα) to test whether platelets derived from scuPA- or uPAT-megakaryocytes would prevent thrombus formation. NSG/VWFR1326H mice exhibited a lower thrombotic burden after carotid artery injury compared with NSG mice unless infused with human platelets or megakaryocytes, whereas intravenous injection of uPA-megakaryocytes generated sufficient uPA-containing human platelets to lyse nascent thrombi. These studies describe the use of in vitro-generated megakaryocytes as a potential platform for delivering uPA or other ectopic proteins within platelet α-granules to sites of vascular injury.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures
Update of
-
Packaging of supplemented urokinase into naked alpha-granules of in vitro -grown megakaryocytes for targeted therapeutic delivery.bioRxiv [Preprint]. 2023 Dec 5:2023.12.05.570278. doi: 10.1101/2023.12.05.570278. bioRxiv. 2023. Update in: Blood Adv. 2024 Jul 23;8(14):3798-3809. doi: 10.1182/bloodadvances.2024012835. PMID: 38106191 Free PMC article. Updated. Preprint.
Similar articles
-
Packaging of supplemented urokinase into naked alpha-granules of in vitro -grown megakaryocytes for targeted therapeutic delivery.bioRxiv [Preprint]. 2023 Dec 5:2023.12.05.570278. doi: 10.1101/2023.12.05.570278. bioRxiv. 2023. Update in: Blood Adv. 2024 Jul 23;8(14):3798-3809. doi: 10.1182/bloodadvances.2024012835. PMID: 38106191 Free PMC article. Updated. Preprint.
-
Increased expression of urokinase plasminogen activator in Quebec platelet disorder is linked to megakaryocyte differentiation.Blood. 2009 Feb 12;113(7):1535-42. doi: 10.1182/blood-2008-08-172338. Epub 2008 Nov 24. Blood. 2009. PMID: 19029443 Free PMC article.
-
A chimeric platelet-targeted urokinase prodrug selectively blocks new thrombus formation.J Clin Invest. 2016 Feb;126(2):483-94. doi: 10.1172/JCI81470. J Clin Invest. 2016. PMID: 26690701 Free PMC article.
-
Quebec platelet disorder: update on pathogenesis, diagnosis, and treatment.Semin Thromb Hemost. 2011 Sep;37(6):713-20. doi: 10.1055/s-0031-1291382. Epub 2011 Nov 18. Semin Thromb Hemost. 2011. PMID: 22102275 Review.
-
Expression of platelet proteins during the in vitro and in vivo differentiation of megakaryocytes and morphological aspects of their maturation.Semin Hematol. 1986 Jan;23(1):43-67. Semin Hematol. 1986. PMID: 3003921 Review. No abstract available.
Cited by
-
The winding road to platelet α-granules.Front Cell Dev Biol. 2025 Apr 16;13:1584059. doi: 10.3389/fcell.2025.1584059. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40309239 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

