Patient-Reported Outcomes From the Phase III HIMALAYA Study of Tremelimumab Plus Durvalumab in Unresectable Hepatocellular Carcinoma
- PMID: 38805668
- PMCID: PMC11315407
- DOI: 10.1200/JCO.23.01462
Patient-Reported Outcomes From the Phase III HIMALAYA Study of Tremelimumab Plus Durvalumab in Unresectable Hepatocellular Carcinoma
Abstract
Purpose: In the phase III HIMALAYA study (ClinicalTrials.gov identifier: NCT03298451) in unresectable hepatocellular carcinoma (uHCC), the Single Tremelimumab Regular Interval Durvalumab (STRIDE) regimen significantly improved overall survival versus sorafenib, and durvalumab monotherapy was noninferior to sorafenib. Patient-reported outcomes (PROs), a secondary outcome from HIMALAYA, are reported here.
Methods: Participants were randomly assigned to receive STRIDE, durvalumab, or sorafenib. PROs were assessed (preplanned secondary outcome) using the European Organization for Research and Treatment of Cancer 30-item Quality of Life Questionnaire and the 18-item HCC module. Time to deterioration (TTD), change from baseline and improvement rate in global health status/quality of life (GHS/QoL), functioning, and disease-related symptoms were analyzed.
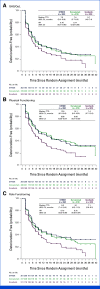
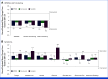
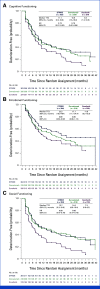
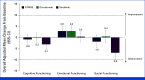
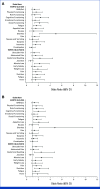
Results: In total, 1,171 participants were randomly assigned to STRIDE (n = 393), durvalumab (n = 389), or sorafenib (n = 389) and were evaluable for PRO assessments. Across treatment arms, compliance rates for PROs were >77% at baseline and >70% overall. Baseline scores were comparable across treatment arms. TTD in GHS/QoL, physical functioning, fatigue, appetite loss, and abdominal pain was numerically longer for both STRIDE and durvalumab versus sorafenib. Clinically meaningful deterioration in PROs was not observed in any treatment arm. However, TTD in nausea and abdominal swelling was numerically longer for STRIDE versus sorafenib, and the likelihood of clinically meaningful improvement in GHS/QoL, role, emotional and social functioning, and disease-related symptoms was greater with STRIDE and durvalumab versus sorafenib. PROs with STRIDE and durvalumab were generally similar.
Conclusion: Compared with sorafenib, STRIDE and durvalumab were associated with clinically meaningful, patient-centered GHS/QoL, functioning, and symptom benefits in people with uHCC. These findings support the benefits of the STRIDE regimen compared with sorafenib for a diverse population reflective of the global uHCC population.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures

References
-
- Sung H, Ferlay J, Siegel RL, et al. : Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 - PubMed
-
- Ferlay J, Ervik M, Lam F, et al. : Global Cancer Observatory: Cancer Today: Liver Factsheet. Lyon, France, International Agency for Research on Cancer, 2020
-
- Bray F, Ferlay J, Soerjomataram I, et al. : Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394-424, 2018 - PubMed
-
- Llovet JM, Kelley RK, Villanueva A, et al. : Hepatocellular carcinoma. Nat Rev Dis Primers 7:6, 2021 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

