Screening embryos for polygenic disease risk: a review of epidemiological, clinical, and ethical considerations
- PMID: 38805697
- PMCID: PMC11369226
- DOI: 10.1093/humupd/dmae012
Screening embryos for polygenic disease risk: a review of epidemiological, clinical, and ethical considerations
Abstract
Background: The genetic composition of embryos generated by in vitro fertilization (IVF) can be examined with preimplantation genetic testing (PGT). Until recently, PGT was limited to detecting single-gene, high-risk pathogenic variants, large structural variants, and aneuploidy. Recent advances have made genome-wide genotyping of IVF embryos feasible and affordable, raising the possibility of screening embryos for their risk of polygenic diseases such as breast cancer, hypertension, diabetes, or schizophrenia. Despite a heated debate around this new technology, called polygenic embryo screening (PES; also PGT-P), it is already available to IVF patients in some countries. Several articles have studied epidemiological, clinical, and ethical perspectives on PES; however, a comprehensive, principled review of this emerging field is missing.
Objective and rationale: This review has four main goals. First, given the interdisciplinary nature of PES studies, we aim to provide a self-contained educational background about PES to reproductive specialists interested in the subject. Second, we provide a comprehensive and critical review of arguments for and against the introduction of PES, crystallizing and prioritizing the key issues. We also cover the attitudes of IVF patients, clinicians, and the public towards PES. Third, we distinguish between possible future groups of PES patients, highlighting the benefits and harms pertaining to each group. Finally, our review, which is supported by ESHRE, is intended to aid healthcare professionals and policymakers in decision-making regarding whether to introduce PES in the clinic, and if so, how, and to whom.
Search methods: We searched for PubMed-indexed articles published between 1/1/2003 and 1/3/2024 using the terms 'polygenic embryo screening', 'polygenic preimplantation', and 'PGT-P'. We limited the review to primary research papers in English whose main focus was PES for medical conditions. We also included papers that did not appear in the search but were deemed relevant.
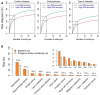
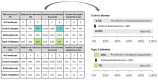
Outcomes: The main theoretical benefit of PES is a reduction in lifetime polygenic disease risk for children born after screening. The magnitude of the risk reduction has been predicted based on statistical modelling, simulations, and sibling pair analyses. Results based on all methods suggest that under the best-case scenario, large relative risk reductions are possible for one or more diseases. However, as these models abstract several practical limitations, the realized benefits may be smaller, particularly due to a limited number of embryos and unclear future accuracy of the risk estimates. PES may negatively impact patients and their future children, as well as society. The main personal harms are an unindicated IVF treatment, a possible reduction in IVF success rates, and patient confusion, incomplete counselling, and choice overload. The main possible societal harms include discarded embryos, an increasing demand for 'designer babies', overemphasis of the genetic determinants of disease, unequal access, and lower utility in people of non-European ancestries. Benefits and harms will vary across the main potential patient groups, comprising patients already requiring IVF, fertile people with a history of a severe polygenic disease, and fertile healthy people. In the United States, the attitudes of IVF patients and the public towards PES seem positive, while healthcare professionals are cautious, sceptical about clinical utility, and concerned about patient counselling.
Wider implications: The theoretical potential of PES to reduce risk across multiple polygenic diseases requires further research into its benefits and harms. Given the large number of practical limitations and possible harms, particularly unnecessary IVF treatments and discarded viable embryos, PES should be offered only within a research context before further clarity is achieved regarding its balance of benefits and harms. The gap in attitudes between healthcare professionals and the public needs to be narrowed by expanding public and patient education and providing resources for informative and unbiased genetic counselling.
Keywords: PGT-P; clinical considerations; disease risk reduction; ethical considerations; polygenic embryo screening; polygenic risk scores; preimplantation genetic testing; statistical genetics.
© The Author(s) 2024. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
S.C. is a paid consultant and owns stock options at MyHeritage; he further declares consulting fees from Infinity X and an (unpaid) collaboration on specific projects with Juno Genetics. The other authors declare no conflicts of interest.
Figures





References
-
- Abu-El-Haija A, Reddi HV, Wand H, Rose NC, Mori M, Qian E, Murray MF, Committee APPG;. ACMG Professional Practice and Guidelines Committee. The clinical application of polygenic risk scores: a points to consider statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med 2023;25:100803. - PubMed
-
- Agar N. Liberal eugenics. Public Aff Q 1998;12:137–155. - PubMed
-
- Al Thani A, Fthenou E, Paparrodopoulos S, Al Marri A, Shi Z, Qafoud F, Afifi N.. Qatar biobank cohort study: study design and first results. Am J Epidemiol 2019;188:1420–1433. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

