Liver Imaging Reporting and Data System Contrast-Enhanced US Nonradiation Treatment Response Assessment Version 2024
- PMID: 38805727
- PMCID: PMC11140523
- DOI: 10.1148/radiol.232369
Liver Imaging Reporting and Data System Contrast-Enhanced US Nonradiation Treatment Response Assessment Version 2024
Abstract
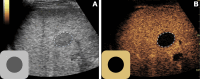
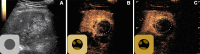
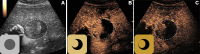
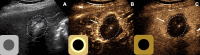
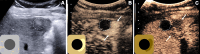
The American College of Radiology Liver Imaging Reporting and Data System (LI-RADS) standardizes the imaging technique, reporting lexicon, disease categorization, and management for patients with or at risk for hepatocellular carcinoma (HCC). LI-RADS encompasses HCC surveillance with US; HCC diagnosis with CT, MRI, or contrast-enhanced US (CEUS); and treatment response assessment (TRA) with CT or MRI. LI-RADS was recently expanded to include CEUS TRA after nonradiation locoregional therapy or surgical resection. This report provides an overview of LI-RADS CEUS Nonradiation TRA v2024, including a lexicon of imaging findings, techniques, and imaging criteria for posttreatment tumor viability assessment. LI-RADS CEUS Nonradiation TRA v2024 takes into consideration differences in the CEUS appearance of viable tumor and posttreatment changes within and in close proximity to a treated lesion. Due to the high sensitivity of CEUS to vascular flow, posttreatment reactive changes commonly manifest as areas of abnormal perilesional enhancement without washout, especially in the first 3 months after treatment. To improve the accuracy of CEUS for nonradiation TRA, different diagnostic criteria are used to evaluate tumor viability within and outside of the treated lesion margin. Broader criteria for intralesional enhancement increase sensitivity for tumor viability detection. Stricter criteria for perilesional enhancement limit miscategorization of posttreatment reactive changes as viable tumor. Finally, the TRA algorithm reconciles intralesional and perilesional tumor viability assessment and assigns a single LI-RADS treatment response (LR-TR) category: LR-TR nonviable, LR-TR equivocal, or LR-TR viable.
© RSNA, 2024 See also the editorial by Milot in this issue.
Conflict of interest statement
Figures

References
-
- Bansal S , Gui J , Merrill C , Wong JK , Burak KW , Wilson SR . Contrast-enhanced US in local ablative therapy and secondary surveillance for hepatocellular carcinoma . RadioGraphics 2019. ; 39 ( 5 ): 1302 – 1322 . - PubMed
-
- Wink MH , Wijkstra H , De La Rosette JJ , Grimbergen CA . Ultrasound imaging and contrast agents: a safe alternative to MRI? Minim Invasive Ther Allied Technol 2006. ; 15 ( 2 ): 93 – 100 . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

