Overcoming Resistance to Immune Checkpoint Blockade in Liver Cancer with Combination Therapy: Stronger Together?
- PMID: 38806159
- PMCID: PMC11245330
- DOI: 10.1055/a-2334-8311
Overcoming Resistance to Immune Checkpoint Blockade in Liver Cancer with Combination Therapy: Stronger Together?
Abstract
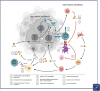
Primary liver cancer, represented mainly by hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (CCA), is one of the most common and deadliest tumors worldwide. While surgical resection or liver transplantation are the best option in early disease stages, these tumors often present in advanced stages and systemic treatment is required to improve survival time. The emergence of immune checkpoint inhibitor (ICI) therapy has had a positive impact especially on the treatment of advanced cancers, thereby establishing immunotherapy as part of first-line treatment in HCC and CCA. Nevertheless, low response rates reflect on the usually cold or immunosuppressed tumor microenvironment of primary liver cancer. In this review, we aim to summarize mechanisms of resistance leading to tumor immune escape with a special focus on the composition of tumor microenvironment in both HCC and CCA, also reflecting on recent important developments in ICI combination therapy. Furthermore, we discuss how combination of ICIs with established primary liver cancer treatments (e.g. multikinase inhibitors and chemotherapy) as well as more complex combinations with state-of-the-art therapeutic concepts may reshape the tumor microenvironment, leading to higher response rates and long-lasting antitumor immunity for primary liver cancer patients.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/).
Conflict of interest statement
F.T.'s lab has received research funding from AstraZeneca, MSD, and Gilead (funding to the institution). Other authors have nothing to declare.
Figures

Similar articles
-
Strategies to Improve the Antitumor Effect of Immunotherapy for Hepatocellular Carcinoma.Front Immunol. 2021 Nov 26;12:783236. doi: 10.3389/fimmu.2021.783236. eCollection 2021. Front Immunol. 2021. PMID: 34899747 Free PMC article. Review.
-
Immune suppressive checkpoint interactions in the tumour microenvironment of primary liver cancers.Br J Cancer. 2022 Jan;126(1):10-23. doi: 10.1038/s41416-021-01453-3. Epub 2021 Aug 16. Br J Cancer. 2022. PMID: 34400801 Free PMC article. Review.
-
Progress of immune checkpoint inhibitors in the treatment of advanced hepatocellular carcinoma.Front Immunol. 2024 Aug 9;15:1455716. doi: 10.3389/fimmu.2024.1455716. eCollection 2024. Front Immunol. 2024. PMID: 39185414 Free PMC article. Review.
-
Exploring the role of the immune microenvironment in hepatocellular carcinoma: Implications for immunotherapy and drug resistance.Elife. 2024 Aug 15;13:e95009. doi: 10.7554/eLife.95009. Elife. 2024. PMID: 39146202 Free PMC article. Review.
-
Advances in Immune Checkpoint Therapy in Hepatocellular Carcinoma.Br J Hosp Med (Lond). 2024 Sep 30;85(9):1-21. doi: 10.12968/hmed.2024.0375. Epub 2024 Sep 30. Br J Hosp Med (Lond). 2024. PMID: 39347660 Review.
Cited by
-
Chronic Inflammation and Immune Dysregulation in Metabolic-Dysfunction-Associated Steatotic Liver Disease Progression: From Steatosis to Hepatocellular Carcinoma.Biomedicines. 2025 May 21;13(5):1260. doi: 10.3390/biomedicines13051260. Biomedicines. 2025. PMID: 40427086 Free PMC article. Review.
-
Spatial‒temporal heterogeneities of liver cancer and the discovery of the invasive zone.Clin Transl Med. 2025 Feb;15(2):e70224. doi: 10.1002/ctm2.70224. Clin Transl Med. 2025. PMID: 39924620 Free PMC article. Review.
-
Tumor-Associated Macrophages: Polarization, Immunoregulation, and Immunotherapy.Cells. 2025 May 19;14(10):741. doi: 10.3390/cells14100741. Cells. 2025. PMID: 40422244 Free PMC article. Review.
-
Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) Impacts Long-Term Outcomes After Curative-Intent Surgery for Hepatocellular Carcinoma.Aliment Pharmacol Ther. 2025 Apr;61(8):1318-1332. doi: 10.1111/apt.70002. Epub 2025 Feb 18. Aliment Pharmacol Ther. 2025. PMID: 39964081 Free PMC article.
References
-
- Sung H, Ferlay J, Siegel R L et al.Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(03):209–249. - PubMed
-
- Forner A, Vidili G, Rengo M, Bujanda L, Ponz-Sarvisé M, Lamarca A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int. 2019;39 01:98–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

