Long-term safety and efficacy of upadacitinib versus adalimumab in patients with rheumatoid arthritis: 5-year data from the phase 3, randomised SELECT-COMPARE study
- PMID: 38806190
- PMCID: PMC11138271
- DOI: 10.1136/rmdopen-2023-004007
Long-term safety and efficacy of upadacitinib versus adalimumab in patients with rheumatoid arthritis: 5-year data from the phase 3, randomised SELECT-COMPARE study
Abstract
Objectives: To assess the safety and efficacy of upadacitinib versus adalimumab from SELECT-COMPARE over 5 years.
Methods: Patients with rheumatoid arthritis and inadequate response to methotrexate were randomised to receive upadacitinib 15 mg once daily, placebo or adalimumab 40 mg every other week, all with concomitant methotrexate. By week 26, patients with insufficient response to randomised treatment were rescued; patients remaining on placebo switched to upadacitinib. Patients completing the 48-week double-blind period could enter a long-term extension. Safety and efficacy were assessed through week 264, with radiographic progression analysed through week 192. Safety was assessed by treatment-emergent adverse events (TEAEs). Efficacy was analysed by randomised group (non-responder imputation (NRI)) or treatment sequence (as observed).
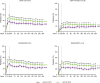
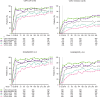
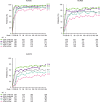
Results: Rates of TEAEs were generally similar with upadacitinib versus adalimumab, although numerically higher rates of herpes zoster, lymphopenia, creatine phosphokinase elevation, hepatic disorder and non-melanoma skin cancer were reported with upadacitinib. Numerically greater proportions of patients randomised to upadacitinib versus adalimumab achieved clinical responses (NRI); Clinical Disease Activity Index remission (≤2.8) and Disease Activity Score based on C reactive protein <2.6 were achieved by 24.6% vs 18.7% (nominal p=0.042) and 31.8% vs 23.2% (nominal p=0.006), respectively. Radiographic progression was numerically lower with continuous upadacitinib versus adalimumab at week 192.
Conclusion: The safety profile of upadacitinib through 5 years was consistent with the known safety profile of upadacitinib, with no new safety risks. Clinical responses were numerically higher with upadacitinib versus adalimumab at 5 years. Upadacitinib demonstrates a favourable benefit-risk profile for long-term rheumatoid arthritis treatment.
Trial registration number: NCT02629159.
Keywords: Antirheumatic Agents; Arthritis, Rheumatoid; Biological Therapy; Inflammation.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RF has received consulting fees and/or grant/research support from AbbVie, Amgen, BI, Biosplice, BMS, Flexion, Galapagos, Galvani, Gilead, GSK, Horizon, Janssen, Lilly, Novartis, Pfizer, Sanofi-Aventis, Selecta, UCB, Viela, Vorso and Vyne and has participated on a data safety monitoring board or advisory board for Kiniksa. JS has received speaking fees, consulting fees and grant/research support from AbbVie, Accord, BMS, Janssen, MSD, Pfizer, Roche, Sandoz and UCB. SKP, XB, NK and YL are employees of AbbVie and may hold stock or options. PD has received speaker fees from AbbVie, Galapagos, Lilly, Nordimed and Thermofischer. LB has received speaking fees, consulting fees and grant/research support from AbbVie, Amgen, BMS, Celgene, Lilly, Fresenius Kabi, Gilead, Janssen, Novartis, Organon, Pfizer, Sanofi-Aventis, Teva and UCB. CGP is an employee and shareholder of Spire Sciences and has served as a consultant for Aclaris, AstraZeneca, Daiichi-Sankyo, Five Prime, Genentech, Gilead, GSK, Istesso, Labcorp, Lilly, Pacira, Paradigm, SetPoint, Sorrento, SynOx and UCB. YT has received speaker fees and/or honoraria from AbbVie, Asahikasei, AstraZeneca, BI, BMS, Chugai, Eisai, Gilead, GSK, Lilly, Pfizer, Taiho and Taisho and research grants from Asahikasei, Chugai, Eisai, Mitsubishi-Tanabe and Taisho. EM has received speaking fees, consulting fees and grant/research support from AbbVie, Amgen, AstraZeneca, BMS, Hi-Bio, Janssen, Lilly, Novartis, Pfizer, Roche, Sandoz and Sanofi, and has received payment for expert testimony from AbbVie.
Figures


References
-
- Burmester GR, Kremer JM, Van den Bosch F, et al. . Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018;391:2503–12. 10.1016/S0140-6736(18)31115-2 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
